Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma
- PMID: 28232723
- PMCID: PMC5333128
- DOI: 10.1038/ncomms14437
Mutant Kras- and p16-regulated NOX4 activation overcomes metabolic checkpoints in development of pancreatic ductal adenocarcinoma
Abstract
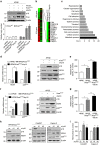
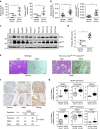
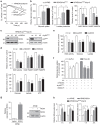
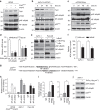
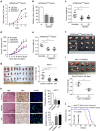
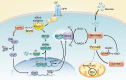
Kras activation and p16 inactivation are required to develop pancreatic ductal adenocarcinoma (PDAC). However, the biochemical mechanisms underlying these double alterations remain unclear. Here we discover that NAD(P)H oxidase 4 (NOX4), an enzyme known to catalyse the oxidation of NAD(P)H, is upregulated when p16 is inactivated by looking at gene expression profiling studies. Activation of NOX4 requires catalytic subunit p22phox, which is upregulated following Kras activation. Both alterations are also detectable in PDAC cell lines and patient specimens. Furthermore, we show that elevated NOX4 activity accelerates oxidation of NADH and supports increased glycolysis by generating NAD+, a substrate for GAPDH-mediated glycolytic reaction, promoting PDAC cell growth. Mechanistically, NOX4 was induced through p16-Rb-regulated E2F and p22phox was induced by KrasG12V-activated NF-κB. In conclusion, we provide a biochemical explanation for the cooperation between p16 inactivation and Kras activation in PDAC development and suggest that NOX4 is a potential therapeutic target for PDAC.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Similar articles
-
PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network.Cancer Discov. 2011 Jul;1(2):158-69. doi: 10.1158/2159-8290.CD-11-0031. Epub 2011 May 23. Cancer Discov. 2011. PMID: 21984975 Free PMC article.
-
KrasG12D-induced IKK2/β/NF-κB activation by IL-1α and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma.Cancer Cell. 2012 Jan 17;21(1):105-20. doi: 10.1016/j.ccr.2011.12.006. Cancer Cell. 2012. PMID: 22264792 Free PMC article.
-
Ribonucleoprotein HNRNPA2B1 interacts with and regulates oncogenic KRAS in pancreatic ductal adenocarcinoma cells.Gastroenterology. 2014 Oct;147(4):882-892.e8. doi: 10.1053/j.gastro.2014.06.041. Epub 2014 Jul 3. Gastroenterology. 2014. PMID: 24998203
-
Critical role of oncogenic KRAS in pancreatic cancer (Review).Mol Med Rep. 2016 Jun;13(6):4943-9. doi: 10.3892/mmr.2016.5196. Epub 2016 Apr 27. Mol Med Rep. 2016. PMID: 27121414 Review.
-
The Roles of Frequently Mutated Genes of Pancreatic Cancer in Regulation of Tumor Microenvironment.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820920969. doi: 10.1177/1533033820920969. Technol Cancer Res Treat. 2020. PMID: 32372692 Free PMC article. Review.
Cited by
-
Nicotinamide adenine dinucleotide phosphate oxidase in pancreatic diseases: Mechanisms and future perspectives.World J Gastroenterol. 2024 Feb 7;30(5):429-439. doi: 10.3748/wjg.v30.i5.429. World J Gastroenterol. 2024. PMID: 38414585 Free PMC article. Review.
-
Oncogenic Kras-Mediated Cytokine CCL15 Regulates Pancreatic Cancer Cell Migration and Invasion through ROS.Cancers (Basel). 2022 Apr 26;14(9):2153. doi: 10.3390/cancers14092153. Cancers (Basel). 2022. PMID: 35565279 Free PMC article.
-
NOX4: a potential therapeutic target for pancreatic cancer and its mechanism.J Transl Med. 2021 Dec 20;19(1):515. doi: 10.1186/s12967-021-03182-w. J Transl Med. 2021. PMID: 34930338 Free PMC article. Review.
-
Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment.Nat Rev Cancer. 2021 Aug;21(8):510-525. doi: 10.1038/s41568-021-00375-9. Epub 2021 Jul 9. Nat Rev Cancer. 2021. PMID: 34244683 Free PMC article. Review.
-
The roles of KRAS in cancer metabolism, tumor microenvironment and clinical therapy.Mol Cancer. 2025 Jan 13;24(1):14. doi: 10.1186/s12943-024-02218-1. Mol Cancer. 2025. PMID: 39806421 Free PMC article. Review.
References
-
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 63, 11–30 (2013). - PubMed
-
- Bardeesy N. & DePinho R. A. Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2, 897–909 (2002). - PubMed
-
- Schutte M. et al.. Abrogation of the Rb/p16 tumor-suppressive pathway in virtually all pancreatic carcinomas. Cancer Res. 57, 3126–3130 (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

