In situ click chemistry generation of cyclooxygenase-2 inhibitors
- PMID: 28232747
- PMCID: PMC5431875
- DOI: 10.1038/s41467-016-0009-6
In situ click chemistry generation of cyclooxygenase-2 inhibitors
Abstract
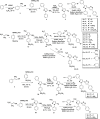
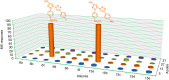
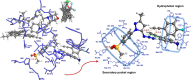
Cyclooxygenase-2 isozyme is a promising anti-inflammatory drug target, and overexpression of this enzyme is also associated with several cancers and neurodegenerative diseases. The amino-acid sequence and structural similarity between inducible cyclooxygenase-2 and housekeeping cyclooxygenase-1 isoforms present a significant challenge to design selective cyclooxygenase-2 inhibitors. Herein, we describe the use of the cyclooxygenase-2 active site as a reaction vessel for the in situ generation of its own highly specific inhibitors. Multi-component competitive-binding studies confirmed that the cyclooxygenase-2 isozyme can judiciously select most appropriate chemical building blocks from a pool of chemicals to build its own highly potent inhibitor. Herein, with the use of kinetic target-guided synthesis, also termed as in situ click chemistry, we describe the discovery of two highly potent and selective cyclooxygenase-2 isozyme inhibitors. The in vivo anti-inflammatory activity of these two novel small molecules is significantly higher than that of widely used selective cyclooxygenase-2 inhibitors.Traditional inflammation and pain relief drugs target both cyclooxygenase 1 and 2 (COX-1 and COX-2), causing severe side effects. Here, the authors use in situ click chemistry to develop COX-2 specific inhibitors with high in vivo anti-inflammatory activity.
Figures





Similar articles
-
Design, synthesis and structure-activity relationship studies of novel and diverse cyclooxygenase-2 inhibitors as anti-inflammatory drugs.J Enzyme Inhib Med Chem. 2014 Dec;29(6):846-67. doi: 10.3109/14756366.2013.864650. Epub 2014 Feb 11. J Enzyme Inhib Med Chem. 2014. PMID: 24517373
-
Synthesis, biological evaluation and docking analysis of 3-methyl-1-phenylchromeno[4,3-c]pyrazol-4(1H)-ones as potential cyclooxygenase-2 (COX-2) inhibitors.Bioorg Med Chem Lett. 2014 Oct 1;24(19):4638-4642. doi: 10.1016/j.bmcl.2014.08.050. Epub 2014 Aug 29. Bioorg Med Chem Lett. 2014. PMID: 25219899
-
Optimization of pyrazole-based compounds with 1,2,4-triazole-3-thiol moiety as selective COX-2 inhibitors cardioprotective drug candidates: Design, synthesis, cyclooxygenase inhibition, anti-inflammatory, ulcerogenicity, cardiovascular evaluation, and molecular modeling studies.Bioorg Chem. 2021 Sep;114:105122. doi: 10.1016/j.bioorg.2021.105122. Epub 2021 Jun 25. Bioorg Chem. 2021. PMID: 34243075
-
Computer aided drug design approaches to develop cyclooxygenase based novel anti-inflammatory and anti-cancer drugs.Curr Pharm Des. 2007;13(34):3505-17. doi: 10.2174/138161207782794275. Curr Pharm Des. 2007. PMID: 18220787 Review.
-
Coumarins scaffolds as COX inhibitors.Bioorg Chem. 2017 Apr;71:146-159. doi: 10.1016/j.bioorg.2017.02.001. Epub 2017 Feb 9. Bioorg Chem. 2017. PMID: 28222891 Review.
Cited by
-
Biomimetic nanoparticles with red blood cell membranes for enhanced photothermal and immunotherapy for tumors.RSC Adv. 2024 Oct 17;14(45):32818-32826. doi: 10.1039/d4ra06965j. eCollection 2024 Oct 17. RSC Adv. 2024. PMID: 39429938 Free PMC article.
-
Development of Fluorescence Imaging Probes for Labeling COX-1 in Live Ovarian Cancer Cells.ACS Med Chem Lett. 2021 Apr 8;12(5):798-804. doi: 10.1021/acsmedchemlett.1c00065. eCollection 2021 May 13. ACS Med Chem Lett. 2021. PMID: 34055228 Free PMC article.
-
Application of Dual Metabarcoding Platforms for the Meso- and Macrozooplankton Taxa in the Ross Sea.Genes (Basel). 2022 May 21;13(5):922. doi: 10.3390/genes13050922. Genes (Basel). 2022. PMID: 35627306 Free PMC article.
-
The Role of Nuclear Medicine for COVID-19: Time to Act Now.J Nucl Med. 2020 Jun;61(6):781-782. doi: 10.2967/jnumed.120.246611. Epub 2020 Apr 17. J Nucl Med. 2020. PMID: 32303597 Free PMC article. No abstract available.
-
A fluorescent target-guided Paal-Knorr reaction.RSC Adv. 2020;10(61):37035-37039. doi: 10.1039/d0ra06962k. Epub 2020 Oct 7. RSC Adv. 2020. PMID: 34262697 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

