Copper Enhances Zinc-Induced Neurotoxicity and the Endoplasmic Reticulum Stress Response in a Neuronal Model of Vascular Dementia
- PMID: 28232787
- PMCID: PMC5299027
- DOI: 10.3389/fnins.2017.00058
Copper Enhances Zinc-Induced Neurotoxicity and the Endoplasmic Reticulum Stress Response in a Neuronal Model of Vascular Dementia
Abstract
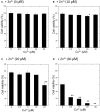
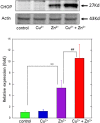
Zinc (Zn), an essential trace element, is secreted by synaptic vesicles during neuronal excitation and plays several critical roles in neuronal information processing. However, excess Zn ion (Zn2+) is neurotoxic and has a causative role in the pathogenesis of vascular dementia. Here, we investigated the molecular mechanism of Zn2+-induced neurotoxicity by using immortalized hypothalamic neurons (GT1-7 cells), which are more vulnerable than other neuronal cells to Zn2+. We examined the effects of other metal ions on the Zn2+-induced neurotoxicity in these cells and found that sub-lethal concentrations of copper ion (Cu2+) markedly exacerbated Zn2+-induced neurotoxicity. The co-administration of Cu2+ and Zn2+ also significantly increased the expression of genes related to the endoplasmic reticulum's stress response, including CHOP, GADD34, and ATF4. Similar to Zn2+, Cu2+ is stored in presynaptic vesicles and secreted during neuronal excitation. Thus, based on our results, we hypothesize here that Cu2+ interacts with Zn2+ in the synapse to synergistically promote neuronal death and significantly influence the pathogenesis of vascular dementia.
Keywords: ER stress; dementia; ischemia; metal–metal interaction; neurotoxicity; synapse.
Figures



Similar articles
-
Nickel Enhances Zinc-Induced Neuronal Cell Death by Priming the Endoplasmic Reticulum Stress Response.Oxid Med Cell Longev. 2019 Jun 17;2019:9693726. doi: 10.1155/2019/9693726. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31316722 Free PMC article.
-
Thioredoxin-albumin fusion protein prevents copper enhanced zinc-induced neurotoxicity via its antioxidative activity.Int J Pharm. 2018 Jan 15;535(1-2):140-147. doi: 10.1016/j.ijpharm.2017.11.012. Epub 2017 Nov 6. Int J Pharm. 2018. PMID: 29122608
-
The molecular mechanisms of zinc neurotoxicity and the pathogenesis of vascular type senile dementia.Int J Mol Sci. 2013 Nov 7;14(11):22067-81. doi: 10.3390/ijms141122067. Int J Mol Sci. 2013. PMID: 24213606 Free PMC article. Review.
-
Zinc, Copper, and Calcium: A Triangle in the Synapse for the Pathogenesis of Vascular-Type Senile Dementia.Biomolecules. 2024 Jun 28;14(7):773. doi: 10.3390/biom14070773. Biomolecules. 2024. PMID: 39062487 Free PMC article. Review.
-
Copper as a Collaborative Partner of Zinc-Induced Neurotoxicity in the Pathogenesis of Vascular Dementia.Int J Mol Sci. 2021 Jul 6;22(14):7242. doi: 10.3390/ijms22147242. Int J Mol Sci. 2021. PMID: 34298862 Free PMC article. Review.
Cited by
-
The Role of Zinc in the Development of Vascular Dementia and Parkinson's Disease and the Potential of Carnosine as Their Therapeutic Agent.Biomedicines. 2024 Jun 11;12(6):1296. doi: 10.3390/biomedicines12061296. Biomedicines. 2024. PMID: 38927502 Free PMC article. Review.
-
Differential protein expression in diverse brain areas of Parkinson's and Alzheimer's disease patients.Sci Rep. 2020 Aug 4;10(1):13149. doi: 10.1038/s41598-020-70174-z. Sci Rep. 2020. PMID: 32753661 Free PMC article.
-
Optimization of a Methodology for Quantification and Removal of Zinc Gives Insights Into the Effect of This Metal on the Stability and Function of the Zinc-Binding Co-chaperone Ydj1.Front Chem. 2019 Jun 11;7:416. doi: 10.3389/fchem.2019.00416. eCollection 2019. Front Chem. 2019. PMID: 31263692 Free PMC article.
-
Emodin inhibits zinc-induced neurotoxicity in neuroblastoma SH-SY5Y cells.Biosci Rep. 2019 May 14;39(5):BSR20182378. doi: 10.1042/BSR20182378. Print 2019 May 31. Biosci Rep. 2019. PMID: 31023967 Free PMC article.
-
Zinc Chloride Exposure Inhibits Brain Acetylcholine Levels, Produces Neurotoxic Signatures, and Diminishes Memory and Motor Activities in Adult Zebrafish.Int J Mol Sci. 2018 Oct 16;19(10):3195. doi: 10.3390/ijms19103195. Int J Mol Sci. 2018. PMID: 30332818 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

