Phase Difference between Model Cortical Areas Determines Level of Information Transfer
- PMID: 28232796
- PMCID: PMC5298997
- DOI: 10.3389/fncom.2017.00006
Phase Difference between Model Cortical Areas Determines Level of Information Transfer
Abstract
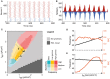
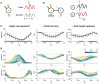
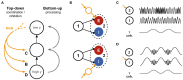
Communication between cortical sites is mediated by long-range synaptic connections. However, these connections are relatively static, while everyday cognitive tasks demand a fast and flexible routing of information in the brain. Synchronization of activity between distant cortical sites has been proposed as the mechanism underlying such a dynamic communication structure. Here, we study how oscillatory activity affects the excitability and input-output relation of local cortical circuits and how it alters the transmission of information between cortical circuits. To this end, we develop model circuits showing fast oscillations by the PING mechanism, of which the oscillatory characteristics can be altered. We identify conditions for synchronization between two brain circuits and show that the level of intercircuit coherence and the phase difference is set by the frequency difference between the intrinsic oscillations. We show that the susceptibility of the circuits to inputs, i.e., the degree of change in circuit output following input pulses, is not uniform throughout the oscillation period and that both firing rate, frequency and power are differentially modulated by inputs arriving at different phases. As a result, an appropriate phase difference between the circuits is critical for the susceptibility windows of the circuits in the network to align and for information to be efficiently transferred. We demonstrate that changes in synchrony and phase difference can be used to set up or abolish information transfer in a network of cortical circuits.
Keywords: PING; communication through coherence; information transfer; multiplexing; oscillations; phase difference; synchrony.
Figures




Similar articles
-
Macroscopic phase resetting-curves determine oscillatory coherence and signal transfer in inter-coupled neural circuits.PLoS Comput Biol. 2019 May 9;15(5):e1007019. doi: 10.1371/journal.pcbi.1007019. eCollection 2019 May. PLoS Comput Biol. 2019. PMID: 31071085 Free PMC article.
-
Phase-locking patterns underlying effective communication in exact firing rate models of neural networks.PLoS Comput Biol. 2022 May 18;18(5):e1009342. doi: 10.1371/journal.pcbi.1009342. eCollection 2022 May. PLoS Comput Biol. 2022. PMID: 35584147 Free PMC article.
-
Flexible information routing by transient synchrony.Nat Neurosci. 2017 Jul;20(7):1014-1022. doi: 10.1038/nn.4569. Epub 2017 May 22. Nat Neurosci. 2017. PMID: 28530664
-
Functional integration across oscillation frequencies by cross-frequency phase synchronization.Eur J Neurosci. 2018 Oct;48(7):2399-2406. doi: 10.1111/ejn.13767. Epub 2017 Dec 2. Eur J Neurosci. 2018. PMID: 29094462 Review.
-
Control of excitatory hierarchical circuits by parvalbumin-FS basket cells in layer 5 of the frontal cortex: insights for cortical oscillations.J Neurophysiol. 2019 Jun 1;121(6):2222-2236. doi: 10.1152/jn.00778.2018. Epub 2019 Apr 17. J Neurophysiol. 2019. PMID: 30995139 Free PMC article. Review.
Cited by
-
Engagement of Pulvino-cortical Feedforward and Feedback Pathways in Cognitive Computations.Neuron. 2019 Jan 16;101(2):321-336.e9. doi: 10.1016/j.neuron.2018.11.023. Epub 2018 Dec 12. Neuron. 2019. PMID: 30553546 Free PMC article.
-
Unified thalamic model generates multiple distinct oscillations with state-dependent entrainment by stimulation.PLoS Comput Biol. 2017 Oct 26;13(10):e1005797. doi: 10.1371/journal.pcbi.1005797. eCollection 2017 Oct. PLoS Comput Biol. 2017. PMID: 29073146 Free PMC article.
-
Attentional selection and communication through coherence: Scope and limitations.PLoS Comput Biol. 2024 Aug 5;20(8):e1011431. doi: 10.1371/journal.pcbi.1011431. eCollection 2024 Aug. PLoS Comput Biol. 2024. PMID: 39102437 Free PMC article.
-
The Orientation Selectivity of Spike-LFP Synchronization in Macaque V1 and V4.Front Comput Neurosci. 2019 Jul 11;13:47. doi: 10.3389/fncom.2019.00047. eCollection 2019. Front Comput Neurosci. 2019. PMID: 31354464 Free PMC article.
-
On the emergence of cognition: from catalytic closure to neuroglial closure.J Biol Phys. 2020 Mar;46(1):95-119. doi: 10.1007/s10867-020-09543-8. Epub 2020 Mar 4. J Biol Phys. 2020. PMID: 32130568 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

