Dexamethasone Inhibits S. aureus-Induced Neutrophil Extracellular Pathogen-Killing Mechanism, Possibly through Toll-Like Receptor Regulation
- PMID: 28232829
- PMCID: PMC5299007
- DOI: 10.3389/fimmu.2017.00060
Dexamethasone Inhibits S. aureus-Induced Neutrophil Extracellular Pathogen-Killing Mechanism, Possibly through Toll-Like Receptor Regulation
Abstract
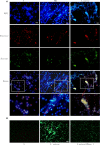
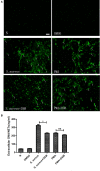
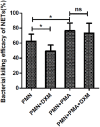
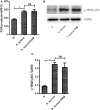
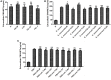
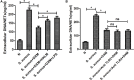
Neutrophils release neutrophil extracellular traps (NETs) in a pathogen-killing process called NETosis. Excessive NETs formation, however, is implicated in disease pathogenesis. Therefore, to understand how NETosis is regulated, we examined the effect of dexamethasone (DXM), an anti-inflammatory drug, on this process and the role of toll-like receptors (TLRs). We stimulated human neutrophils with phorbol 12-myristate 13-acetate (PMA) or Staphylococcus aureus (S. aureus) and quantified NETs formation. We also examined the effect of DXM on the bactericidal effect of NETs and the role of reactive oxygen species (ROS) and nuclear factor (NF)-κB in DXM-regulated NETosis. DXM significantly inhibited S. aureus-induced NETosis and extracellular bacterial killing. ROS production and NF-κB activation were not involved in DXM-regulated NETosis. TLR2 and TLR4, but not TLR5 or TLR6, modified S. aureus-induced NETs formation. Neither DXM nor TLRs were involved in PMA-induced NETosis. Furthermore, TLR2 and TLR4 agonists rescued DXM-inhibited NETosis, and neither TLR2 nor TLR4 antagonists could further inhibit NETosis reduction induced by DXM, indicating that DXM may inhibit NETosis by regulating TLR2 and TLR4. In conclusion, the mechanisms of S. aureus- and PMA-induced NETosis are different. DXM decreases NETs formation independently of oxidant production and NF-κB phosphorylation and possibly via a TLR-dependent mechanism.
Keywords: PMA; S. aureus; TLRs; dexamethasone; neutrophil extracellular traps.
Figures
Similar articles
-
(+)-Borneol inhibits the generation of reactive oxygen species and neutrophil extracellular traps induced by phorbol-12-myristate-13-acetate.Front Pharmacol. 2022 Nov 7;13:1023450. doi: 10.3389/fphar.2022.1023450. eCollection 2022. Front Pharmacol. 2022. PMID: 36419617 Free PMC article.
-
Hypertonic Saline Suppresses NADPH Oxidase-Dependent Neutrophil Extracellular Trap Formation and Promotes Apoptosis.Front Immunol. 2018 Mar 8;9:359. doi: 10.3389/fimmu.2018.00359. eCollection 2018. Front Immunol. 2018. PMID: 29593709 Free PMC article.
-
Acetylsalicylic Acid Promotes Corneal Epithelium Migration by Regulating Neutrophil Extracellular Traps in Alkali Burn.Front Immunol. 2020 Oct 15;11:551057. doi: 10.3389/fimmu.2020.551057. eCollection 2020. Front Immunol. 2020. PMID: 33178183 Free PMC article.
-
Neutrophil Extracellular Traps and Their Possible Implications in Ocular Herpes Infection.Pathogens. 2023 Jan 29;12(2):209. doi: 10.3390/pathogens12020209. Pathogens. 2023. PMID: 36839481 Free PMC article. Review.
-
An exploratory look at NETosis in atherosclerosis.Intern Emerg Med. 2017 Feb;12(1):13-22. doi: 10.1007/s11739-016-1543-2. Epub 2016 Sep 21. Intern Emerg Med. 2017. PMID: 27655025 Review.
Cited by
-
Targeted delivery of an anti-inflammatory corticosteroid to Ly6C/G-positive cells abates severity of influenza A symptoms.Proc Natl Acad Sci U S A. 2022 Oct 25;119(43):e2211065119. doi: 10.1073/pnas.2211065119. Epub 2022 Oct 17. Proc Natl Acad Sci U S A. 2022. PMID: 36252038 Free PMC article.
-
Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression.Adv Biol Regul. 2020 Aug;77:100741. doi: 10.1016/j.jbior.2020.100741. Epub 2020 Jul 4. Adv Biol Regul. 2020. PMID: 32773102 Free PMC article. Review.
-
Neutrophil extracellular traps may have a dual role in Pseudomonas aeruginosa keratitis.Eur J Clin Microbiol Infect Dis. 2021 Jan;40(1):169-180. doi: 10.1007/s10096-020-04023-2. Epub 2020 Sep 1. Eur J Clin Microbiol Infect Dis. 2021. PMID: 32875519
-
Neutrophil extracellular traps activate lung fibroblast to induce polymyositis-related interstitial lung diseases via TLR9-miR-7-Smad2 pathway.J Cell Mol Med. 2020 Jan;24(2):1658-1669. doi: 10.1111/jcmm.14858. Epub 2019 Dec 10. J Cell Mol Med. 2020. PMID: 31821687 Free PMC article.
-
DNA Sensor IFI204 Contributes to Host Defense Against Staphylococcus aureus Infection in Mice.Front Immunol. 2019 Mar 18;10:474. doi: 10.3389/fimmu.2019.00474. eCollection 2019. Front Immunol. 2019. PMID: 30936875 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

