Selectively Inducing Cancer Cell Death by Intracellular Enzyme-Instructed Self-Assembly (EISA) of Dipeptide Derivatives
- PMID: 28233466
- PMCID: PMC5550337
- DOI: 10.1002/adhm.201601400
Selectively Inducing Cancer Cell Death by Intracellular Enzyme-Instructed Self-Assembly (EISA) of Dipeptide Derivatives
Abstract
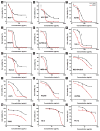
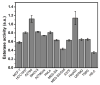
Tight ligand-receptor binding, paradoxically, is a major root of drug resistance in cancer chemotherapy. To address this problem, instead of using conventional inhibitors or ligands, this paper focuses on the development of a novel process-enzyme-instructed self-assembly (EISA)-to kill cancer cells selectively. Here it is demonstrated that EISA as an intracellular process to generate nanofibrils of short peptides for selectively inhibiting cancer cell proliferation, including drug resistant ones. As the process that turns the non-self-assembling precursors into the self-assembling peptides upon the catalysis of carboxylesterases (CES), EISA occurs intracellularly to selectively inhibit a range of cancer cells that exhibit relatively high CES activities. More importantly, EISA inhibits drug resistant cancer cells (e.g., triple negative breast cancer cells (HCC1937) and platinum-resistant ovarian cells (SKOV3, A2780cis)). With the IC50 values of 28-80 and 25-44 µg mL-1 of l- and d-dipeptide precursors against cancer cells, respectively, EISA is innocuous to normal cells. Moreover, using coculture of cancer and normal cells, the selectivity of EISA is validated against cancer cells. Besides revealing that intracellular EISA cause apoptosis or necroptosis to kill the cancer cells, this work illustrates a new approach to amplify the enzymatic difference between cancer and normal cells and to expand the pool of drug candidates for potentially overcoming drug resistance in cancer therapy.
Keywords: anticancer; drug-resistance; enzyme; selectivity; self-assembly.
© 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

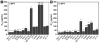
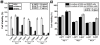

Similar articles
-
Kinetic Analysis of Nanostructures Formed by Enzyme-Instructed Intracellular Assemblies against Cancer Cells.ACS Nano. 2018 Apr 24;12(4):3804-3815. doi: 10.1021/acsnano.8b01016. Epub 2018 Mar 21. ACS Nano. 2018. PMID: 29537820 Free PMC article.
-
Enzyme-Instructed Intracellular Molecular Self-Assembly to Boost Activity of Cisplatin against Drug-Resistant Ovarian Cancer Cells.Angew Chem Int Ed Engl. 2015 Nov 2;54(45):13307-11. doi: 10.1002/anie.201507157. Epub 2015 Sep 14. Angew Chem Int Ed Engl. 2015. PMID: 26365295 Free PMC article.
-
Enzyme-Instructed Assembly and Disassembly Processes for Targeting Downregulation in Cancer Cells.J Am Chem Soc. 2017 Mar 22;139(11):3950-3953. doi: 10.1021/jacs.7b00070. Epub 2017 Mar 13. J Am Chem Soc. 2017. PMID: 28257192 Free PMC article.
-
Enzyme-instructed self-assembly: a multistep process for potential cancer therapy.Bioconjug Chem. 2015 Jun 17;26(6):987-99. doi: 10.1021/acs.bioconjchem.5b00196. Epub 2015 May 14. Bioconjug Chem. 2015. PMID: 25933032 Free PMC article. Review.
-
Enzyme-Instructed Supramolecular Self-Assembly with Anticancer Activity.Adv Mater. 2019 Nov;31(45):e1804814. doi: 10.1002/adma.201804814. Epub 2018 Nov 16. Adv Mater. 2019. PMID: 30444545 Review.
Cited by
-
Intracellular Enzyme-Instructed Self-Assembly of Peptides (IEISAP) for Biomedical Applications.Molecules. 2022 Oct 4;27(19):6557. doi: 10.3390/molecules27196557. Molecules. 2022. PMID: 36235094 Free PMC article. Review.
-
Designing bioresponsive nanomaterials for intracellular self-assembly.Nat Rev Chem. 2022 May;6(5):320-338. doi: 10.1038/s41570-022-00373-x. Epub 2022 Apr 1. Nat Rev Chem. 2022. PMID: 37117928 Free PMC article. Review.
-
Self-Assembling Ability Determines the Activity of Enzyme-Instructed Self-Assembly for Inhibiting Cancer Cells.J Am Chem Soc. 2017 Nov 1;139(43):15377-15384. doi: 10.1021/jacs.7b07147. Epub 2017 Oct 23. J Am Chem Soc. 2017. PMID: 28990765 Free PMC article.
-
Supramolecular biofunctional materials.Biomaterials. 2017 Jun;129:1-27. doi: 10.1016/j.biomaterials.2017.03.014. Epub 2017 Mar 12. Biomaterials. 2017. PMID: 28319779 Free PMC article. Review.
-
Kinetic Analysis of Nanostructures Formed by Enzyme-Instructed Intracellular Assemblies against Cancer Cells.ACS Nano. 2018 Apr 24;12(4):3804-3815. doi: 10.1021/acsnano.8b01016. Epub 2018 Mar 21. ACS Nano. 2018. PMID: 29537820 Free PMC article.
References
-
- Hanahan D, Weinberg RA. Cell. 2011;144:646. - PubMed
-
- Dong HD, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu GF, Tamada K, Lennon VA, Celis E, Chen LP. Nat Med. 2002;8:793. - PubMed
-
- Di Virgilio F, Vuerich M. Auton Neurosci-basic. 2015;191:117. - PubMed
-
- Wilczyński JR, Nowak M. Cancer Immunology. Springer; 2015. p. 413.
- Adams SF, Benencia F. Future Oncol. 2015;11:1293. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

