Targeting Phenotypically Tolerant Mycobacterium tuberculosis
- PMID: 28233509
- PMCID: PMC5367488
- DOI: 10.1128/microbiolspec.TBTB2-0031-2016
Targeting Phenotypically Tolerant Mycobacterium tuberculosis
Abstract
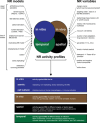
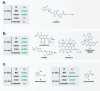
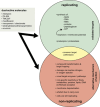
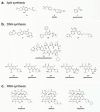
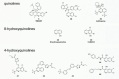
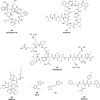
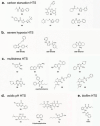
While the immune system is credited with averting tuberculosis in billions of individuals exposed to Mycobacterium tuberculosis, the immune system is also culpable for tempering the ability of antibiotics to deliver swift and durable cure of disease. In individuals afflicted with tuberculosis, host immunity produces diverse microenvironmental niches that support suboptimal growth, or complete growth arrest, of M. tuberculosis. The physiological state of nonreplication in bacteria is associated with phenotypic drug tolerance. Many of these host microenvironments, when modeled in vitro by carbon starvation, complete nutrient starvation, stationary phase, acidic pH, reactive nitrogen intermediates, hypoxia, biofilms, and withholding streptomycin from the streptomycin-addicted strain SS18b, render M. tuberculosis profoundly tolerant to many of the antibiotics that are given to tuberculosis patients in clinical settings. Targeting nonreplicating persisters is anticipated to reduce the duration of antibiotic treatment and rate of posttreatment relapse. Some promising drugs to treat tuberculosis, such as rifampin and bedaquiline, only kill nonreplicating M. tuberculosisin vitro at concentrations far greater than their minimal inhibitory concentrations against replicating bacilli. There is an urgent demand to identify which of the currently used antibiotics, and which of the molecules in academic and corporate screening collections, have potent bactericidal action on nonreplicating M. tuberculosis. With this goal, we review methods of high-throughput screening to target nonreplicating M. tuberculosis and methods to progress candidate molecules. A classification based on structures and putative targets of molecules that have been reported to kill nonreplicating M. tuberculosis revealed a rich diversity in pharmacophores.
Figures


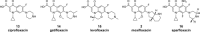

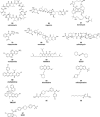
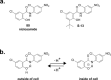
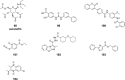
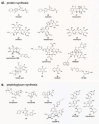
References
-
- Hobby GL, Meyer K, Chaffee E. 1942. Observations on the mechanism of action of penicillin. Exp Biol Med 50:281–285. 10.3181/00379727-50-13773 - DOI
-
- Bigger J. 1944. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244:497–500. 10.1016/S0140-6736(00)74210-3 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

