Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: A position paper
- PMID: 28233641
- PMCID: PMC5385293
- DOI: 10.1016/j.clinph.2017.01.003
Guiding transcranial brain stimulation by EEG/MEG to interact with ongoing brain activity and associated functions: A position paper
Abstract
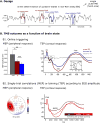
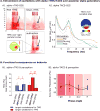
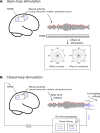
Non-invasive transcranial brain stimulation (NTBS) techniques have a wide range of applications but also suffer from a number of limitations mainly related to poor specificity of intervention and variable effect size. These limitations motivated recent efforts to focus on the temporal dimension of NTBS with respect to the ongoing brain activity. Temporal patterns of ongoing neuronal activity, in particular brain oscillations and their fluctuations, can be traced with electro- or magnetoencephalography (EEG/MEG), to guide the timing as well as the stimulation settings of NTBS. These novel, online and offline EEG/MEG-guided NTBS-approaches are tailored to specifically interact with the underlying brain activity. Online EEG/MEG has been used to guide the timing of NTBS (i.e., when to stimulate): by taking into account instantaneous phase or power of oscillatory brain activity, NTBS can be aligned to fluctuations in excitability states. Moreover, offline EEG/MEG recordings prior to interventions can inform researchers and clinicians how to stimulate: by frequency-tuning NTBS to the oscillation of interest, intrinsic brain oscillations can be up- or down-regulated. In this paper, we provide an overview of existing approaches and ideas of EEG/MEG-guided interventions, and their promises and caveats. We point out potential future lines of research to address challenges.
Keywords: Brain oscillations; Electroencephalography; Magnetoencephalography; Non-invasive transcranial brain stimulation (NTBS); Temporally guided NTBS.
Copyright © 2017 International Federation of Clinical Neurophysiology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
GT has received honoraria as editor from Wiley Publishers. FF is the lead inventor of IP filed by UNC. The clinical studies performed in the Frohlich Lab have received a designation as conflict of interest with administrative considerations. FF is the founder, CSO, and majority owner of Pulvinar Neuro LLC. HRS has served on a scientific advisory board for Lundbeck A/S, Valby Denmark, and has received honoraria as speaker from Biogen Idec, Denmark A/S, Genzyme, Denmark and MerckSerono, Denmark, has received honoraria as editor from Elsevier Publishers, Amsterdam, The Netherlands and Springer Publishing, Stuttgart, Germany, has received travel support from MagVenture, Denmark, and has received a research grant from Biogen-idec. UZ has received personal fees from Biogen Idec GmbH, Bayer Vital GmbH, Bristol Myers Squibb GmbH, CorTec GmbH, Medtronic GmbH, and grants from Biogen Idec GmbH, Servier, and Janssen Pharmaceuticals NV, outside of the submitted work. CSH has received honoraria as editor from Elsevier Publishers, Amsterdam. The remaining authors have no conflicts of interest.
Figures


Comment in
-
Smart brain stimulation.Clin Neurophysiol. 2017 May;128(5):839-840. doi: 10.1016/j.clinph.2017.02.002. Epub 2017 Feb 15. Clin Neurophysiol. 2017. PMID: 28283357 No abstract available.
-
How effective is transcranial direct current stimulation?Lancet. 2024 Jun 22;403(10445):2688-2689. doi: 10.1016/S0140-6736(24)00634-2. Lancet. 2024. PMID: 38908869 No abstract available.
References
-
- Alekseichuk I, Turi Z, Amador de Lara G, Antal A, Paulus W. Spatial Working Memory in Humans Depends on Theta and High Gamma Synchronization in the Prefrontal Cortex. Curr Biol. 2016;26:1513–21. - PubMed
-
- Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1:97–105. - PubMed
-
- Artola A, Bröcher S, Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990;347:69–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

