Spectroelectrochemical analysis of the mechanism of (photo)electrochemical hydrogen evolution at a catalytic interface
- PMID: 28233785
- PMCID: PMC5333116
- DOI: 10.1038/ncomms14280
Spectroelectrochemical analysis of the mechanism of (photo)electrochemical hydrogen evolution at a catalytic interface
Abstract
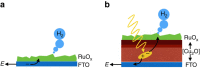
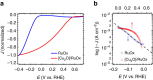
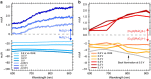
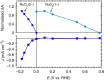
Multi-electron heterogeneous catalysis is a pivotal element in the (photo)electrochemical generation of solar fuels. However, mechanistic studies of these systems are difficult to elucidate by means of electrochemical methods alone. Here we report a spectroelectrochemical analysis of hydrogen evolution on ruthenium oxide employed as an electrocatalyst and as part of a cuprous oxide-based photocathode. We use optical absorbance spectroscopy to quantify the densities of reduced ruthenium oxide species, and correlate these with current densities resulting from proton reduction. This enables us to compare directly the catalytic function of dark and light electrodes. We find that hydrogen evolution is second order in the density of active, doubly reduced species independent of whether these are generated by applied potential or light irradiation. Our observation of a second order rate law allows us to distinguish between the most common reaction paths and propose a mechanism involving the homolytic reductive elimination of hydrogen.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
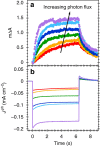
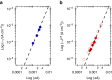
References
-
- Wang Q. et al.. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 1–3 (2016). - PubMed
-
- Haro M. et al.. Toward stable solar hydrogen generation using organic photoelectrochemical cells. J. Phys. Chem. C 119, 6488–6494 (2015).
-
- Willkomm J. et al.. Dye-sensitised semiconductors modified with molecular catalysts for light-driven H2 production. Chem. Soc. Rev. 45, 9–23 (2016). - PubMed
-
- Cook T. R. et al.. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 110, 6474–6502 (2010). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

