Sponge-associated bacteria mineralize arsenic and barium on intracellular vesicles
- PMID: 28233852
- PMCID: PMC5333131
- DOI: 10.1038/ncomms14393
Sponge-associated bacteria mineralize arsenic and barium on intracellular vesicles
Abstract
Arsenic and barium are ubiquitous environmental toxins that accumulate in higher trophic-level organisms. Whereas metazoans have detoxifying organs to cope with toxic metals, sponges lack organs but harbour a symbiotic microbiome performing various functions. Here we examine the potential roles of microorganisms in arsenic and barium cycles in the sponge Theonella swinhoei, known to accumulate high levels of these metals. We show that a single sponge symbiotic bacterium, Entotheonella sp., constitutes the arsenic- and barium-accumulating entity within the host. These bacteria mineralize both arsenic and barium on intracellular vesicles. Our results indicate that Entotheonella sp. may act as a detoxifying organ for its host.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



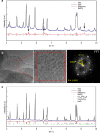

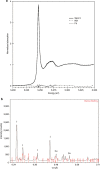

References
-
- Oremland R. S. & Stolz J. F. The ecology of arsenic. Science 300, 939–944 (2003). - PubMed
-
- Dehairs F., Chesselet R. & Jedwab J. Discrete suspended particles of barite and the barium cycle in the open ocean. Earth Planet. Sci. Lett. 49, 528–550 (1980).
-
- Neff J. M. Ecotoxicology of arsenic in the marine environment. Environ. Toxicol. Chem. 16, 917–927 (1997).
-
- Menzie C. A., Southworth B., Stephenson G. & Feisthauer N. The importance of understanding the chemical form of a metal in the environment: the case of barium sulfate (barite). Hum. Ecol. Risk Assess. 14, 974–991 (2008).
-
- Slyemi D. & Bonnefoy V. How prokaryotes deal with arsenic. Environ. Microbiol. Rep. 4, 571–586 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

