Axon contact-driven Schwann cell dedifferentiation
- PMID: 28233923
- PMCID: PMC5395415
- DOI: 10.1002/glia.23131
Axon contact-driven Schwann cell dedifferentiation
Abstract
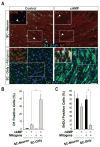
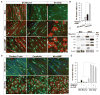
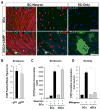
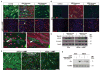
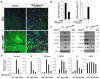
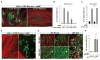
Mature Schwann cells (SCs) retain dedifferentiation potential throughout adulthood. Still, how dedifferentiation occurs remains uncertain. Results from a variety of cell-based assays using in vitro cultured cAMP-differentiated and myelinating SCs revealed the existence of a novel dedifferentiating activity expressed on the surface of dorsal root ganglion (DRG) axons. This activity had the capacity to prevent SC differentiation and elicit dedifferentiation through direct SC-axon contact. Evidence is provided showing that a rapid loss of myelinating SC markers concomitant to proliferation occurred even in the presence of elevated cAMP, a signal that is required to drive and maintain a differentiated state. The dedifferentiating activity was a membrane-bound protein found exclusively in DRG neurons, as judged by its subcellular partitioning, sensitivity to proteolytic degradation and cell-type specificity, and remained active even after disruption of cellular organization. It differed from the membrane-anchored neuregulin-1 isoforms that are responsible for axon contact-induced SC proliferation and exerted its action independently of mitogenic signaling emanating from receptor tyrosine kinases and mitogen-activated protein kinases such as ERK and JNK. Interestingly, dedifferentiation occurred without concomitant changes in the expression of Krox-20, a transcriptional enhancer of myelination, and c-Jun, an inhibitor of myelination. In sum, our data indicated the existence of cell surface axon-derived signals that override pro-differentiating cues, drive dedifferentiation and allow SCs to proliferate in response to axonal mitogens. This axonal signal may negatively regulate myelination at the onset or reversal of the differentiated state. GLIA 2017;65:851-863.
Keywords: cAMP; cell signaling; cell-cell interactions; differentiation; dorsal root ganglion neurons; in vitro systems; myelination; proliferation.
© 2017 Wiley Periodicals, Inc.
Figures






Similar articles
-
Schwann cell dedifferentiation is independent of mitogenic signaling and uncoupled to proliferation: role of cAMP and JNK in the maintenance of the differentiated state.J Biol Chem. 2010 Oct 1;285(40):31024-36. doi: 10.1074/jbc.M110.116970. Epub 2010 Jul 15. J Biol Chem. 2010. PMID: 20634285 Free PMC article.
-
Requirement of cAMP signaling for Schwann cell differentiation restricts the onset of myelination.PLoS One. 2015 Feb 23;10(2):e0116948. doi: 10.1371/journal.pone.0116948. eCollection 2015. PLoS One. 2015. PMID: 25705874 Free PMC article.
-
Opposing roles of PKA and EPAC in the cAMP-dependent regulation of schwann cell proliferation and differentiation [corrected].PLoS One. 2013 Dec 11;8(12):e82354. doi: 10.1371/journal.pone.0082354. eCollection 2013. PLoS One. 2013. PMID: 24349260 Free PMC article.
-
Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation.J Peripher Nerv Syst. 2008 Jun;13(2):122-35. doi: 10.1111/j.1529-8027.2008.00168.x. J Peripher Nerv Syst. 2008. PMID: 18601657 Review.
-
Cross-talk between growth factor and purinergic signalling regulates Schwann cell proliferation.Novartis Found Symp. 2006;276:162-75; discussion 175-80, 233-7, 275-81. Novartis Found Symp. 2006. PMID: 16805429 Review.
Cited by
-
Human hair keratins promote the regeneration of peripheral nerves in a rat sciatic nerve crush model.J Mater Sci Mater Med. 2019 Jul 4;30(7):82. doi: 10.1007/s10856-019-6283-1. J Mater Sci Mater Med. 2019. PMID: 31273463 Free PMC article.
-
Neurofibroma Development in Neurofibromatosis Type 1: Insights from Cellular Origin and Schwann Cell Lineage Development.Cancers (Basel). 2022 Sep 17;14(18):4513. doi: 10.3390/cancers14184513. Cancers (Basel). 2022. PMID: 36139671 Free PMC article. Review.
-
Systemic loss of Sarm1 protects Schwann cells from chemotoxicity by delaying axon degeneration.Commun Biol. 2020 Jan 30;3(1):49. doi: 10.1038/s42003-020-0776-9. Commun Biol. 2020. PMID: 32001778 Free PMC article.
-
The role and mechanism of Schwann cells in the repair of peripheral nerve injury.Cell Tissue Res. 2025 Apr;400(1):81-95. doi: 10.1007/s00441-025-03957-3. Epub 2025 Feb 15. Cell Tissue Res. 2025. PMID: 39954051 Review.
-
Schwann Cell Cultures: Biology, Technology and Therapeutics.Cells. 2020 Aug 6;9(8):1848. doi: 10.3390/cells9081848. Cells. 2020. PMID: 32781699 Free PMC article. Review.
References
-
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

