AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression
- PMID: 28234358
- PMCID: PMC5423107
- DOI: 10.1038/cdd.2017.14
AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression
Abstract
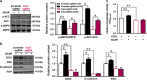
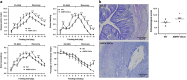
Impairment in gut epithelial integrity and barrier function is associated with many diseases. The homeostasis of intestinal barrier is based on a delicate regulation of epithelial proliferation and differentiation. AMP-activated protein kinase (AMPK) is a master regulator of energy metabolism, and cellular metabolites are intrinsically involved in epigenetic modifications governing cell differentiation. We aimed to evaluate the regulatory role of AMPK on intestinal epithelial development and barrier function. In this study, AMPK activator (AICAR) improved the barrier function of Caco-2 cells as indicated by increased transepithelial electrical resistance and reduced paracellular FITC-dextran permeability; consistently, AICAR enhanced epithelial differentiation and tight junction formation. Transfection of Caco-2 cells with AMPK WT plasmid, which enhances AMPK activity, improved epithelial barrier function and epithelial differentiation, while K45R (AMPK dominant negative mutant) impaired; these changes were correlated with the expression of caudal type homeobox 2 (CDX2), the key transcription factor committing cells to intestinal epithelial lineage. CDX2 deficiency abolished intestinal differentiation promoted by AMPK activation. Mechanistically, AMPK inactivation was associated with polycomb repressive complex 2 regulated enrichment of H3K27me3, the inhibitory histone modification, and lysine-specific histone demethylase-1-mediated reduction of H3K4me3, a permissive histone modification. Those histone modifications provide a mechanistic link between AMPK and CDX2 expression. Consistently, epithelial AMPK knockout in vivo reduced CDX2 expression, impaired intestinal barrier function, integrity and ultrastructure of tight junction, and epithelial cell migration, promoted intestinal proliferation and exaggerated dextran sulfate sodium-induced colitis. In summary, AMPK enhances intestinal barrier function and epithelial differentiation via promoting CDX2 expression, which is partially mediated by altered histone modifications in the Cdx2 promoter.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 2009; 71: 241–260. - PubMed
-
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9: 799–809. - PubMed
-
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8: 774–785. - PubMed
-
- Meddings J. The significance of the gut barrier in disease. Gut 2008; 57: 438–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

