Clinical significance of 11-oxygenated androgens
- PMID: 28234803
- PMCID: PMC5819755
- DOI: 10.1097/MED.0000000000000334
Clinical significance of 11-oxygenated androgens
Abstract
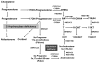
Purpose of review: The adrenal gland is considered a source of weak androgens, such as dehydroepiandrosterone, dehydroepiandrosterone sulfate, and androstenedione. Emerging evidence proposes a set of 11-oxygenated 19-carbon (11oxC19) adrenal-derived steroids as clinically important androgens. Such steroids include 11β-hydroxyandrostenedione, 11-ketoandrostenedione, 11β-hydroxytestosterone, and 11-ketotestosterone. The present review will discuss the synthesis, androgenic activity, and clinical implications of the 11oxC19 steroids.
Recent findings: The clinical relevance of the 11oxC19 steroids resides in two key characteristics: the synthesis of all 11oxC19 originates predominantly in the adrenal cortex, and 11-ketotestosterone and its 5α-reduced metabolite, 11-ketodihydrotestosterone are potent agonists of the human androgen receptor, similar to the classic androgens testosterone and dihydrotestosterone, respectively. Recent studies have demonstrated higher than normal circulating levels of 11oxC19 steroids in patients with 21-hydroxylase deficiency and in polycystic ovary syndrome. The 11oxC19 steroids are also thought to contribute to castration-resistant prostate cancer progression. In addition, the 11oxC19 steroids might have clinical implications in adrenarche and postmenopausal women.
Summary: Future prospective studies are needed to establish the clinical utility of the 11oxC19 steroids for individualized patient care. Preliminary data suggest that these biomarkers hold promise to improve the evaluation and management of androgen excess disorders.
Figures
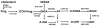
Similar articles
-
Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency.Eur J Endocrinol. 2016 May;174(5):601-9. doi: 10.1530/EJE-15-1181. Epub 2016 Feb 10. Eur J Endocrinol. 2016. PMID: 26865584 Free PMC article.
-
11-Oxygenated Androgens Are Biomarkers of Adrenal Volume and Testicular Adrenal Rest Tumors in 21-Hydroxylase Deficiency.J Clin Endocrinol Metab. 2017 Aug 1;102(8):2701-2710. doi: 10.1210/jc.2016-3989. J Clin Endocrinol Metab. 2017. PMID: 28472487 Free PMC article.
-
Profiling adrenal 11β-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC2-MS/MS quantification of 11β-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone.J Steroid Biochem Mol Biol. 2017 Feb;166:54-67. doi: 10.1016/j.jsbmb.2016.06.009. Epub 2016 Jun 21. J Steroid Biochem Mol Biol. 2017. PMID: 27345701
-
Back where it belongs: 11β-hydroxyandrostenedione compels the re-assessment of C11-oxy androgens in steroidogenesis.Mol Cell Endocrinol. 2021 Apr 5;525:111189. doi: 10.1016/j.mce.2021.111189. Epub 2021 Feb 2. Mol Cell Endocrinol. 2021. PMID: 33539964 Review.
-
A new dawn for androgens: Novel lessons from 11-oxygenated C19 steroids.Mol Cell Endocrinol. 2017 Feb 5;441:76-85. doi: 10.1016/j.mce.2016.08.014. Epub 2016 Aug 9. Mol Cell Endocrinol. 2017. PMID: 27519632 Review.
Cited by
-
Morphologic and Molecular Characterization of Adrenals and Adrenal Rest Affected by Congenital Adrenal Hyperplasia.Front Endocrinol (Lausanne). 2021 Sep 20;12:730947. doi: 10.3389/fendo.2021.730947. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34616364 Free PMC article.
-
11-Oxygenated C19 steroids are the predominant androgens responsible for hyperandrogenemia in Cushing's disease.Eur J Endocrinol. 2022 Sep 29;187(5):663-673. doi: 10.1530/EJE-22-0320. Print 2022 Nov 1. Eur J Endocrinol. 2022. PMID: 36074938 Free PMC article.
-
Association of dehydroepiandrosterone sulfate, birth size, adiposity and cardiometabolic risk factors in 7-year-old children.Pediatr Res. 2022 Jun;91(7):1897-1905. doi: 10.1038/s41390-021-01706-0. Epub 2021 Aug 20. Pediatr Res. 2022. PMID: 34417562
-
Genetically predicted serum metabolites mediate the association between inflammatory proteins and polycystic ovary syndrome: a Mendelian randomization study.Front Med (Lausanne). 2024 Dec 3;11:1433612. doi: 10.3389/fmed.2024.1433612. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39691364 Free PMC article.
-
Salivary Testosterone, Androstenedione and 11-Oxygenated 19-Carbon Concentrations Differ by Age and Sex in Children.Clin Endocrinol (Oxf). 2025 Sep;103(3):338-350. doi: 10.1111/cen.15258. Epub 2025 May 20. Clin Endocrinol (Oxf). 2025. PMID: 40390455 Free PMC article.
References
-
- Turcu AF, Nanba AT, Chomic R, Upadhyay SK, Giordano TJ, Shields JJ, et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016 May;174(5):601–9. This cross-sectional study compared 38 patients (19 men) with classic 21OHD, age 3–59, and 38 sex- and age-matched controls and found that four 11oxC19 steroids were 3 to 4-fold higher in patients with 21OHD. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

