Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial
- PMID: 28235027
- PMCID: PMC5342261
- DOI: 10.1371/journal.ppat.1006182
Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial
Abstract
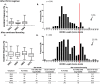
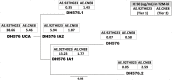
The canary pox vector and gp120 vaccine (ALVAC-HIV and AIDSVAX B/E gp120) in the RV144 HIV-1 vaccine trial conferred an estimated 31% vaccine efficacy. Although the vaccine Env AE.A244 gp120 is antigenic for the unmutated common ancestor of V1V2 broadly neutralizing antibody (bnAbs), no plasma bnAb activity was induced. The RV305 (NCT01435135) HIV-1 clinical trial was a placebo-controlled randomized double-blinded study that assessed the safety and efficacy of vaccine boosting on B cell repertoires. HIV-1-uninfected RV144 vaccine recipients were reimmunized 6-8 years later with AIDSVAX B/E gp120 alone, ALVAC-HIV alone, or a combination of ALVAC-HIV and AIDSVAX B/E gp120 in the RV305 trial. Env-specific post-RV144 and RV305 boost memory B cell VH mutation frequencies increased from 2.9% post-RV144 to 6.7% post-RV305. The vaccine was well tolerated with no adverse events reports. While post-boost plasma did not have bnAb activity, the vaccine boosts expanded a pool of envelope CD4 binding site (bs)-reactive memory B cells with long third heavy chain complementarity determining regions (HCDR3) whose germline precursors and affinity matured B cell clonal lineage members neutralized the HIV-1 CRF01 AE tier 2 (difficult to neutralize) primary isolate, CNE8. Electron microscopy of two of these antibodies bound with near-native gp140 trimers showed that they recognized an open conformation of the Env trimer. Although late boosting of RV144 vaccinees expanded a novel pool of neutralizing B cell clonal lineages, we hypothesize that boosts with stably closed trimers would be necessary to elicit antibodies with greater breadth of tier 2 HIV-1 strains.
Trial registration: ClinicalTrials.gov NCT01435135.
Conflict of interest statement
The ALVAC-HIV canarypox vector is a Sanofi Pasteur patented product. JT and SP are employed by Sanofi Pasteur.
Figures


References
-
- Gray GE, Allen M, Moodie Z, Churchyard G, Bekker LG, et al. (2011) Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis 11: 507–515. 10.1016/S1473-3099(11)70098-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

