Human Alpha-1-Antitrypsin (hAAT) therapy reduces renal dysfunction and acute tubular necrosis in a murine model of bilateral kidney ischemia-reperfusion injury
- PMID: 28235038
- PMCID: PMC5325207
- DOI: 10.1371/journal.pone.0168981
Human Alpha-1-Antitrypsin (hAAT) therapy reduces renal dysfunction and acute tubular necrosis in a murine model of bilateral kidney ischemia-reperfusion injury
Abstract
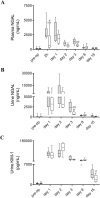
Several lines of evidence have demonstrated the anti-inflammatory and cytoprotective effects of alpha-1-antitrypsin (AAT), the major serum serine protease inhibitor. The aim of the present study was to investigate the effects of human AAT (hAAT) monotherapy during the early and recovery phase of ischemia-induced acute kidney injury. Mild renal ischemia-reperfusion (I/R) injury was induced in male C57Bl/6 mice by bilateral clamping of the renal artery and vein for 20 min. hAAT (80 mg/kg, Prolastin®) was administered daily intraperitoneally (i.p.) from day -1 until day 7 after surgery. Control animals received the same amount of human serum albumin (hAlb). Plasma, urine and kidneys were collected at 2h, 1, 2, 3, 8 and 15 days after reperfusion for histological and biochemical analysis. hAAT partially preserved renal function and tubular integrity after induction of bilateral kidney I/R injury, which was accompanied with reduced renal influx of macrophages and a significant decrease of neutrophil gelatinase-associated lipocalin (NGAL) protein levels in urine and plasma. During the recovery phase, hAAT significantly decreased kidney injury molecule-1 (KIM-1) protein levels in urine but showed no significant effect on renal fibrosis. Although the observed effect size of hAAT administration was limited and therefore the clinical relevance of our findings should be evaluated carefully, these data support the potential of this natural protein to ameliorate ischemic and inflammatory conditions.
Conflict of interest statement
Figures




Similar articles
-
Utility of urinary tubular markers for monitoring chronic tubulointerstitial injury after ischemia-reperfusion.Nephrology (Carlton). 2018 Apr;23(4):308-316. doi: 10.1111/nep.12998. Nephrology (Carlton). 2018. PMID: 28063188
-
Impact of a Single Dose of Alpha-1-Antitrypsin in a Rat Model of Bilateral Kidney Ischemia Reperfusion Injury.J Surg Res. 2024 Jul;299:179-187. doi: 10.1016/j.jss.2024.04.023. Epub 2024 May 17. J Surg Res. 2024. PMID: 38759334
-
Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation.Circulation. 2000 Sep 19;102(12):1420-6. doi: 10.1161/01.cir.102.12.1420. Circulation. 2000. PMID: 10993862
-
Potential molecular therapy for acute renal failure.Cleve Clin J Med. 1993 Mar-Apr;60(2):166-8. doi: 10.3949/ccjm.60.2.166. Cleve Clin J Med. 1993. PMID: 8443951 Review.
-
Animal models of acute tubular necrosis.Curr Opin Crit Care. 2002 Dec;8(6):526-34. doi: 10.1097/00075198-200212000-00008. Curr Opin Crit Care. 2002. PMID: 12454537 Review.
Cited by
-
Nephrotic syndrome secondary to alpha-1 antitrypsin deficiency.BMJ Case Rep. 2021 Mar 5;14(3):e240288. doi: 10.1136/bcr-2020-240288. BMJ Case Rep. 2021. PMID: 33674298 Free PMC article.
-
Knockdown of ELF4 aggravates renal injury in ischemia/reperfusion mice through promotion of pyroptosis, inflammation, oxidative stress, and endoplasmic reticulum stress.BMC Mol Cell Biol. 2023 Jul 20;24(1):22. doi: 10.1186/s12860-023-00485-2. BMC Mol Cell Biol. 2023. PMID: 37474923 Free PMC article.
-
Urine proteomics of primary membranous nephropathy using nanoscale liquid chromatography tandem mass spectrometry analysis.Clin Proteomics. 2018 Feb 7;15:5. doi: 10.1186/s12014-018-9183-3. eCollection 2018. Clin Proteomics. 2018. PMID: 29445323 Free PMC article.
-
Alpha-1-antitrypsin in cell and organ transplantation.Am J Transplant. 2018 Jul;18(7):1589-1595. doi: 10.1111/ajt.14756. Epub 2018 Apr 24. Am J Transplant. 2018. PMID: 29607607 Free PMC article. Review.
-
Fibrinolytic Serine Proteases, Therapeutic Serpins and Inflammation: Fire Dancers and Firestorms.Front Cardiovasc Med. 2021 Mar 25;8:648947. doi: 10.3389/fcvm.2021.648947. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 33869309 Free PMC article. Review.
References
-
- Carell RW. The molecular structure and pathology of alpha 1-antitrypsin. Lung. 1990;168 Suppl: 530–4. - PubMed
-
- Massi G, Chiarelli C. Alpha 1-antitrypsin: molecular structure and the Pi system. Acta Paediatr. 1994;393: 1–4. - PubMed
-
- Wenger RH, Rolfs A, Marti HH, Bauer C, Gassmann M. Hypoxia, a novel inducer of acute phase gene expression in a human hepatoma cell line. J Biol Chem. 1995;270(46): 27865–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

