Altered vasoreactivity in neonatal rats with pulmonary hypertension associated with bronchopulmonary dysplasia: Implication of both eNOS phosphorylation and calcium signaling
- PMID: 28235094
- PMCID: PMC5325597
- DOI: 10.1371/journal.pone.0173044
Altered vasoreactivity in neonatal rats with pulmonary hypertension associated with bronchopulmonary dysplasia: Implication of both eNOS phosphorylation and calcium signaling
Abstract
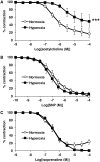
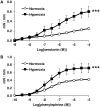
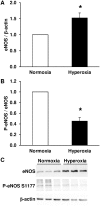
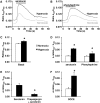
Bronchopulmonary dysplasia (BPD) consists of an arrest of pulmonary vascular and alveolar growth, with persistent hypoplasia of the pulmonary microvasculature and alveolar simplification. In 25 to 40% of the cases, BPD is complicated by pulmonary hypertension (BPD-PH) that significantly increases the risk of morbidity. In vivo studies suggest that increased pulmonary vascular tone could contribute to late PH in BPD. Nevertheless, an alteration in vasoreactivity as well as the mechanisms involved remain to be confirmed. The purpose of this study was thus to assess changes in pulmonary vascular reactivity in a murine model of BPD-PH. Newborn Wistar rats were exposed to either room air (normoxia) or 90% O2 (hyperoxia) for 14 days. Exposure to hyperoxia induced the well-known features of BPD-PH such as elevated right ventricular systolic pressure, right ventricular hypertrophy, pulmonary vascular remodeling and decreased pulmonary vascular density. Intrapulmonary arteries from hyperoxic pups showed decreased endothelium-dependent relaxation to acetylcholine without any alteration of relaxation to the NO-donor sodium nitroprusside. This functional alteration was associated with a decrease of lung eNOS phosphorylation at the Ser1177 activating site. In pups exposed to hyperoxia, serotonin and phenylephrine induced exacerbated contractile responses of intrapulmonary arteries as well as intracellular calcium response in pulmonary arterial smooth muscle cells (PASMC). Moreover, the amplitude of the store-operated Ca2+ entry (SOCE), induced by store depletion using a SERCA inhibitor, was significantly greater in PASMC from hyperoxic pups. Altogether, hyperoxia-induced BPD-PH alters the pulmonary arterial reactivity, with effects on both endothelial and smooth muscle functions. Reduced activating eNOS phosphorylation and enhanced Ca2+ signaling likely account for alterations of pulmonary arterial reactivity.
Conflict of interest statement
Figures

References
-
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164: 1971–1980. 10.1164/ajrccm.164.10.2101140 - DOI - PubMed
-
- Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003;8: 73–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

