Structural and biochemical studies on Vibrio cholerae Hsp31 reveals a novel dimeric form and Glutathione-independent Glyoxalase activity
- PMID: 28235098
- PMCID: PMC5325305
- DOI: 10.1371/journal.pone.0172629
Structural and biochemical studies on Vibrio cholerae Hsp31 reveals a novel dimeric form and Glutathione-independent Glyoxalase activity
Abstract
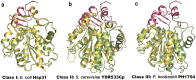
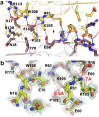
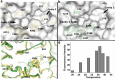
Vibrio cholerae experiences a highly hostile environment at human intestine which triggers the induction of various heat shock genes. The hchA gene product of V. cholerae O395, referred to a hypothetical intracellular protease/amidase VcHsp31, is one such stress-inducible homodimeric protein. Our current study demonstrates that VcHsp31 is endowed with molecular chaperone, amidopeptidase and robust methylglyoxalase activities. Through site directed mutagenesis coupled with biochemical assays on VcHsp31, we have confirmed the role of residues in the vicinity of the active site towards amidopeptidase and methylglyoxalase activities. VcHsp31 suppresses the aggregation of insulin in vitro in a dose dependent manner. Through crystal structures of VcHsp31 and its mutants, grown at various temperatures, we demonstrate that VcHsp31 acquires two (Type-I and Type-II) dimeric forms. Type-I dimer is similar to EcHsp31 where two VcHsp31 monomers associate in eclipsed manner through several intersubunit hydrogen bonds involving their P-domains. Type-II dimer is a novel dimeric organization, where some of the intersubunit hydrogen bonds are abrogated and each monomer swings out in the opposite directions centering at their P-domains, like twisting of wet cloth. Normal mode analysis (NMA) of Type-I dimer shows similar movement of the individual monomers. Upon swinging, a dimeric surface of ~400Å2, mostly hydrophobic in nature, is uncovered which might bind partially unfolded protein substrates. We propose that, in solution, VcHsp31 remains as an equilibrium mixture of both the dimers. With increase in temperature, transformation to Type-II form having more exposed hydrophobic surface, occurs progressively accounting for the temperature dependent increase of chaperone activity of VcHsp31.
Conflict of interest statement
Figures






Similar articles
-
Cloning, expression, purification, crystallization and preliminary X-ray analysis of the 31 kDa Vibrio cholerae heat-shock protein VcHsp31.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Nov 1;67(Pt 11):1382-5. doi: 10.1107/S1744309111032970. Epub 2011 Oct 27. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011. PMID: 22102237 Free PMC article.
-
A new native EcHsp31 structure suggests a key role of structural flexibility for chaperone function.Protein Sci. 2004 Jan;13(1):269-77. doi: 10.1110/ps.03399604. Protein Sci. 2004. PMID: 14691241 Free PMC article.
-
Crystal structure and kinetic studies of a tetrameric type II β-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae.Acta Crystallogr D Biol Crystallogr. 2015 Dec 1;71(Pt 12):2449-56. doi: 10.1107/S1399004715018635. Epub 2015 Nov 26. Acta Crystallogr D Biol Crystallogr. 2015. PMID: 26627652
-
Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation.Biochem Soc Trans. 2003 Dec;31(Pt 6):1343-8. doi: 10.1042/bst0311343. Biochem Soc Trans. 2003. PMID: 14641060 Review.
-
An evolutionary approach to the design of glutathione-linked enzymes.Chem Biol Interact. 1998 Apr 24;111-112:15-21. doi: 10.1016/s0009-2797(97)00147-6. Chem Biol Interact. 1998. PMID: 9679539 Review.
Cited by
-
Structural and functional studies of SAV0551 from Staphylococcus aureus as a chaperone and glyoxalase III.Biosci Rep. 2017 Nov 17;37(6):BSR20171106. doi: 10.1042/BSR20171106. Print 2017 Dec 22. Biosci Rep. 2017. PMID: 29046369 Free PMC article.
-
The origin of esterase activity of Parkinson's disease causative factor DJ-1 implied by evolutionary trace analysis of its prokaryotic homolog HchA.J Biol Chem. 2024 Jul;300(7):107476. doi: 10.1016/j.jbc.2024.107476. Epub 2024 Jun 13. J Biol Chem. 2024. PMID: 38879013 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

