Gut-Brain Cross-Talk in Metabolic Control
- PMID: 28235194
- PMCID: PMC5839146
- DOI: 10.1016/j.cell.2017.01.025
Gut-Brain Cross-Talk in Metabolic Control
Abstract
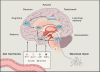
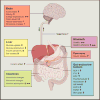
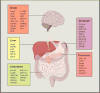
Because human energy metabolism evolved to favor adiposity over leanness, the availability of palatable, easily attainable, and calorically dense foods has led to unprecedented levels of obesity and its associated metabolic co-morbidities that appear resistant to traditional lifestyle interventions. However, recent progress identifying the molecular signaling pathways through which the brain and the gastrointestinal system communicate to govern energy homeostasis, combined with emerging insights on the molecular mechanisms underlying successful bariatric surgery, gives reason to be optimistic that novel precision medicines that mimic, enhance, and/or modulate gut-brain signaling can have unprecedented potential for stopping the obesity and type 2 diabetes pandemics.
Keywords: appetite; bariatric surgery; brain; diabetes; energy balance; gut; metabolic syndrome; obesity; pharmacology; satiety.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Aiello L, Wheeler P. The expensive-tissue hypothesis: the brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36:199–221.
-
- Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SC. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450:106–109. - PubMed
-
- Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8:308–320. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

