The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function
- PMID: 28235408
- PMCID: PMC5324200
- DOI: 10.1186/s12906-017-1585-7
The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function
Abstract
Background: Lianhuaqingwen Capsule (LH-C) is a traditional Chinese medicine (TCM) formula used to treat respiratory tract infectious diseases in Chinese. The aim of this study was to determine the antiviral activity of LH-C and its immunomodulatory effects on viral infection.
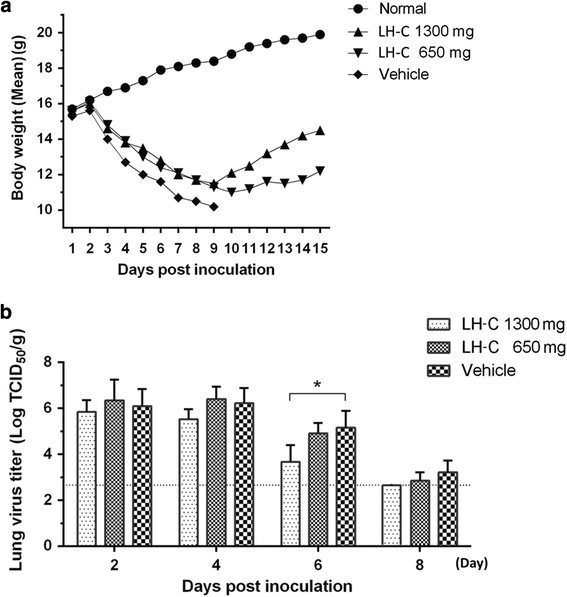
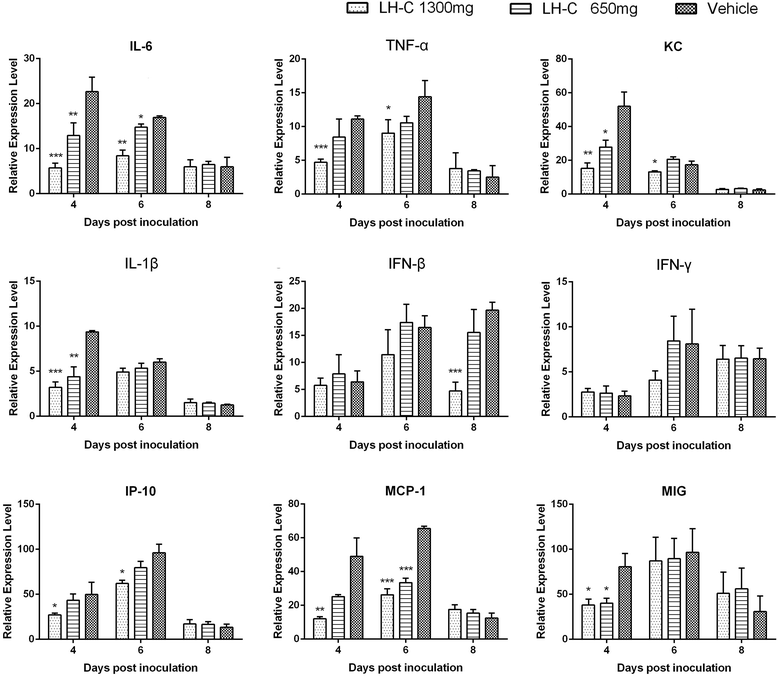
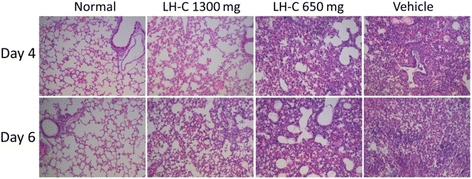
Method: The in vitro cytotoxicity and antiviral activity of LH-C was determined by MTT and Plaque reduction assays. Time course study under single-cycle virus growth conditions were used to determine which stage of viral replication was blocked. The effect of LH-C on the nuclear export of the viral nucleoprotein was examined using an indirect immunofluorescence assay. The regulation to different signaling transduction events and cytokine/chemokine expression of LH-C was evaluated using Western blotting and real-time RT-PCR. After virus inoculation, BALB/c mice were administered with LH-C of different concentrations for 5 days. Body-weight, viral titers and lung pathology of the mice were measured, the level of inflammatory cytokines were also examined using real-time RT-PCR.
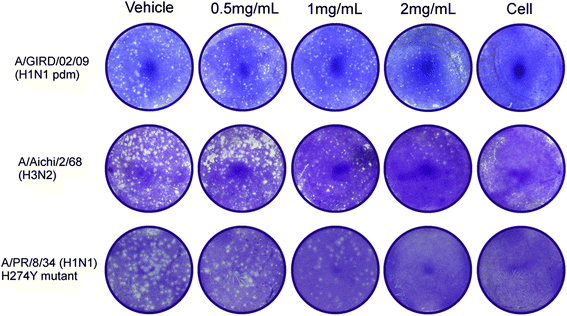
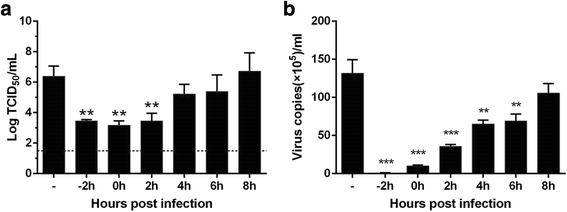
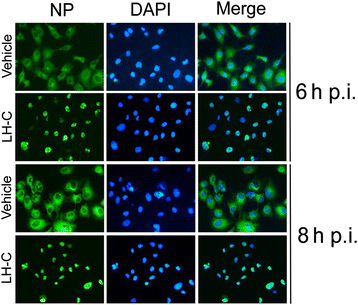
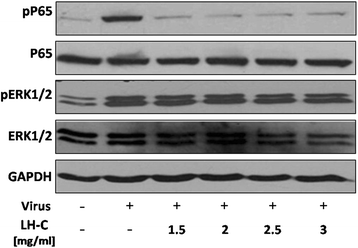
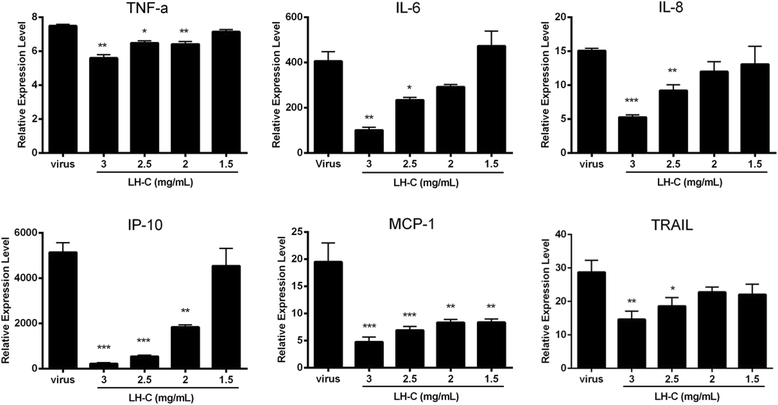
Results: LH-C inhibited the proliferation of influenza viruses of various strain in vitro, with the 50% inhibitory concentration (IC50) ranging from 0.35 to 2 mg/mL. LH-C blocked the early stages (0-2 h) of virus infection, it also suppressed virus-induced NF-kB activation and alleviated virus-induced gene expression of IL-6, IL-8, TNF-a, IP-10, and MCP-1 in a dose-dependent manner. LH-C treatment efficiently impaired the nuclear export of the viral RNP. A decrease of the viral titers in the lungs of mice were observed in groups administered with LH-C. The level of inflammatory cytokines were also decreased in the early stages of infection.
Conclusions: LH-C, as a TCM prescription, exerts broad-spectrum effects on a series of influenza viruses, including the newly emerged H7N9, and particularly regulates the immune response of virus infection. Thus, LH-C might be a promising option for treating influenza virus infection.
Keywords: Antiviral; Immuno-regulation; Influenza virus; Lianhuaqingwen capsule.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

