Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma
- PMID: 28235764
- PMCID: PMC5741820
- DOI: 10.1158/0008-5472.CAN-16-2310
Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma
Abstract
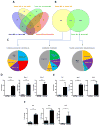
In glioblastoma (GBM), tumor-associated macrophages (TAM) represent up to one half of the cells of the tumor mass, including both infiltrating macrophages and resident brain microglia. In an effort to delineate the temporal and spatial dynamics of TAM composition during gliomagenesis, we used genetically engineered and GL261-induced mouse models in combination with CX3CR1GFP/WT;CCR2RFP/WT double knock-in mice. Using this approach, we demonstrated that CX3CR1LoCCR2Hi monocytes were recruited to the GBM, where they transitioned to CX3CR1HiCCR2Lo macrophages and CX3CR1HiCCR2- microglia-like cells. Infiltrating macrophages/monocytes constituted approximately 85% of the total TAM population, with resident microglia accounting for the approximately 15% remaining. Bone marrow-derived infiltrating macrophages/monocytes were recruited to the tumor early during GBM initiation, where they localized preferentially to perivascular areas. In contrast, resident microglia were localized mainly to peritumoral regions. RNA-sequencing analyses revealed differential gene expression patterns unique to infiltrating and resident cells, suggesting unique functions for each TAM population. Notably, limiting monocyte infiltration via genetic Ccl2 reduction prolonged the survival of tumor-bearing mice. Our findings illuminate the unique composition and functions of infiltrating and resident myeloid cells in GBM, establishing a rationale to target infiltrating cells in this neoplasm. Cancer Res; 77(9); 2266-78. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
The authors declare no Conflict of Interests.
Figures

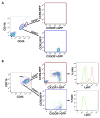
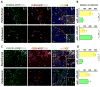
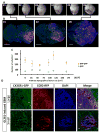
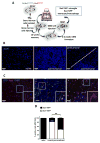
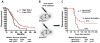
References
-
- Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–9. - PubMed
-
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

