Localization of the gate and selectivity filter of the full-length P2X7 receptor
- PMID: 28235784
- PMCID: PMC5358401
- DOI: 10.1073/pnas.1610414114
Localization of the gate and selectivity filter of the full-length P2X7 receptor
Abstract
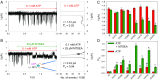
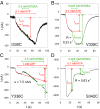
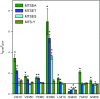
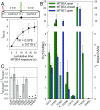
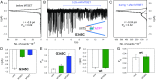
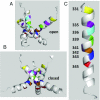
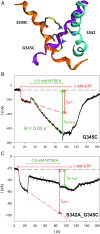
The P2X7 receptor (P2X7R) belongs to the P2X family of ATP-gated cation channels. P2X7Rs are expressed in epithelial cells, leukocytes, and microglia, and they play important roles in immunological and inflammatory processes. P2X7Rs are obligate homotrimers, with each subunit having two transmembrane helices, TM1 and TM2. Structural and functional data regarding the P2X2 and P2X4 receptors indicate that the central trihelical TM2 bundle forms the intrinsic transmembrane channel of P2X receptors. Here, we studied the accessibility of single cysteines substituted along the pre-TM2 and TM2 helix (residues 327-357) of the P2X7R using as readouts (i) the covalent maleimide fluorescence accessibility of the surface-bound P2X7R and (ii) covalent modulation of macroscopic and single-channel currents using extracellularly and intracellularly applied methanethiosulfonate (MTS) reagents. We found that the channel opening extends from the pre-TM2 region through the outer half of the trihelical TM2 channel. Covalently adducted MTS ethylammonium+ (MTSEA+) strongly increased the probability that the channel was open by delaying channel closing of seven of eight responsive human P2X7R (hP2X7R) mutants. Structural modeling, as supported by experimental probing, suggested that resulting intraluminal hydrogen bonding interactions stabilize the open-channel state. The additional decrease in single-channel conductance by MTSEA+ in five of seven positions identified Y336, S339, L341C, Y343, and G345 as the narrowest part of the channel lumen. The gate and ion-selectivity filter of the P2X7R could be colocalized at and around residue S342. None of our results provided any evidence for dilation of the hP2X7R channel on sustained stimulation with ATP4.
Keywords: P2X7 receptor; P2X7 receptor homology model; cysteine-scanning accessibility mutagenesis; single-channel conductance; single-channel open probability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cockcroft S, Gomperts BD. ATP induces nucleotide permeability in rat mast cells. Nature. 1979;279(5713):541–542. - PubMed
-
- Di Virgilio F. The P2Z purinoceptor: An intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16(11):524–528. - PubMed
-
- Rassendren F, et al. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272(9):5482–5486. - PubMed
-
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272(5262):735–738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

