Acute effects of glucagon-like peptide-1, GLP-19-36 amide, and exenatide on mesenteric blood flow, cardiovascular parameters, and biomarkers in healthy volunteers
- PMID: 28235974
- PMCID: PMC5328764
- DOI: 10.14814/phy2.13102
Acute effects of glucagon-like peptide-1, GLP-19-36 amide, and exenatide on mesenteric blood flow, cardiovascular parameters, and biomarkers in healthy volunteers
Abstract
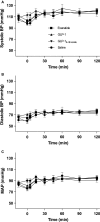
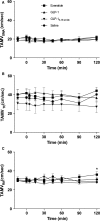
Glucagon-like peptide-1 (GLP-1, GLP-17-36amide) and its sister peptide glucagon-like peptide 2 (GLP-2) influence numerous intestinal functions and GLP-2 greatly increases intestinal blood flow. We hypothesized that GLP-1 also stimulates intestinal blood flow and that this would impact on the overall digestive and cardiovascular effects of the hormone. To investigate the influence of GLP-1 receptor agonism on mesenteric and renal blood flow and cardiovascular parameters, we carried out a double-blinded randomized clinical trial. A total of eight healthy volunteers received high physiological subcutaneous injections of GLP-1, GLP-19-36 amide (bioactive metabolite), exenatide (stable GLP-1 agonist), or saline on four separate days. Blood flow in mesenteric, celiac, and renal arteries was measured by Doppler ultrasound. Blood pressure, heart rate, cardiac output, and stroke volume were measured continuously using an integrated system. Plasma was analyzed for glucose, GLP-1 (intact and total), exenatide and Pancreatic polypeptide (PP), and serum for insulin and C-peptide. Neither GLP-1, GLP-19-36 amide, exenatide nor saline elicited any changes in blood flow parameters in the mesenteric or renal arteries. GLP-1 significantly increased heart rate (two-way ANOVA, injection [P = 0.0162], time [P = 0.0038], and injection × time [P = 0.082]; Tukey post hoc GLP-1 vs. saline and GLP-19-36amide [P < 0.011]), and tended to increase cardiac output and decrease stroke volume compared to GLP-19-36 amide and saline. Blood pressures were not affected. As expected, glucose levels fell and insulin secretion increased after infusion of both GLP-1 and exenatide.
Keywords: Exenatide; glucagon like‐peptide‐1; mesenteric blood flow.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures




References
-
- Asmar, A. , Simonsen L., Asmar M., Madsbad S., Holst J. J., Frandsen E., et al. 2015. Renal extraction and acute effects of glucagon‐like peptide‐1 on central and renal hemodynamics in healthy men. Am. J. Physiol. Endocrinol. Metab. 308:E641–E649. - PubMed
-
- Ban, K. , Noyan‐Ashraf M. H., Hoefer J., Bolz S.‐S., Drucker D. J., and Husain M.. 2008. Cardioprotective and vasodilatory actions of glucagon‐like peptide 1 receptor are mediated through both glucagon‐like peptide 1 receptor‐dependent and ‐independent pathways. Circulation 117:2340–2350. - PubMed
-
- Bremholm, L. , Hornum M., Henriksen B. M., Larsen S., and Holst J. J.. 2009. Glucagon‐like peptide‐2 increases mesenteric blood flow in humans. Scand. J. Gastroenterol. 44:314–319. - PubMed
-
- Bremholm, L. , Hornum M., Andersen U. B., Hartmann B., Holst J. J., and Jeppesen P. B.. 2011. The effect of glucagon‐like peptide‐2 on mesenteric blood flow and cardiac parameters in end‐jejunostomy short bowel patients. Regul. Pept. 168:32–38. - PubMed
-
- Buhl, T. , Thim L., Kofod H., Orskov C., Harling H., and Holst J. J.. 1988. Naturally occurring products of proglucagon 111‐160 in the porcine and human small intestine. J. Biol. Chem. 263:8621–8624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

