Use of concomitant inhaled corticosteroids: pooled data from two phase III studies of aclidinium plus formoterol in COPD
- PMID: 28235977
- PMCID: PMC5434772
- DOI: 10.1038/s41533-016-0009-3
Use of concomitant inhaled corticosteroids: pooled data from two phase III studies of aclidinium plus formoterol in COPD
Abstract
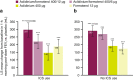
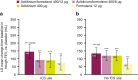
Bronchodilator therapy is the backbone of the management of chronic obstructive pulmonary disease. In some patients, inhaled corticosteroids can be prescribed in combination with bronchodilators. Through a subgroup analysis of pooled data from two large phase III clinical trials of bronchodilator therapy according to concomitant inhaled corticosteroid use (user vs. non-user), we sought to evaluate the clinical benefit of adding inhaled corticosteroids to dual bronchodilator therapy in chronic obstructive pulmonary disease. The primary focus of this analysis of pooled data from the phase III ACLIFORM and AUGMENT studies was to evaluate the efficacy of aclidinium/formoterol on lung function stratified by inhaled corticosteroid use. We found that lung-function end points were significantly improved regardless of concomitant inhaled corticosteroid use among patients treated with the dual bronchodilator aclidinium/formoterol 400/12 µg twice daily compared with placebo and both monotherapies. Together with the previously reported observations that aclidinium/formoterol 400/12 µg reduces exacerbations vs. placebo in inhaled corticosteroid users and improves dyspnoea compared to monotherapy in inhaled corticosteroid non-users, these data suggest that both groups achieve lung function improvements, which translates to different clinical benefits depending on whether or not a patient is receiving concomitant inhaled corticosteroids.CHRONIC LUNG DISEASE: 'TRIPLE' THERAPY COULD PROVE BENEFICIAL: A dual bronchodilator therapy taken together with corticosteroid inhalers may benefit patients with severe chronic lung disease. Bronchodilator drugs relax the lungs and widen airways in patients with chronic obstructive pulmonary disease (COPD). While recent studies have shown that a dual bronchodilator therapy containing aclidinium and formoterol significantly improves lung function in COPD, little is known about combining the dual therapy with inhaled corticosteroids (ICSs). Anthony D'Urzo at the University of Toronto, Canada, and co-workers analysed data from 3394 patients with COPD undergoing dual therapy trials. Of these, 1180 were already taking ICSs. The team compared symptoms in the ICS group with those not taking ICSs. The dual therapy improved lung function across both groups regardless of ICS use, though patients gained different clinical benefits depending on ICS use and disease severity.
Figures
References
-
- European Medicines Agency. Duaklir® Genuair™ (aclidinium bromide / formoterol fumarate dihydrate), http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici..., Accessed 28 November 2016.
-
- Health Canada. Health Canada Regulatory Decision Summary: DUAKLIR GENUAIR, http://www.hc-sc.gc.ca/dhp-mps/prodpharma/rds-sdr/drug-med/rds_sdr_duakl..., Accessed 28 November 2016.
-
- Australian Register of Therapeutic Goods. Public ARTG Summary BRIMICA GENUAIR 340/12 aclidinium bromide and eformoterol fumarate dihydrate powder for inhalation dry powder inhaler, https://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStor..., Accessed 28 November 2016.
-
- Singh D, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm. Med. 2014;14:178–189. doi: 10.1186/1471-2466-14-178. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

