Histone H3 and H4 acetylation patterns are more dynamic than those of DNA methylation in Brachypodium distachyon embryos during seed maturation and germination
- PMID: 28236006
- PMCID: PMC5610208
- DOI: 10.1007/s00709-017-1088-x
Histone H3 and H4 acetylation patterns are more dynamic than those of DNA methylation in Brachypodium distachyon embryos during seed maturation and germination
Abstract
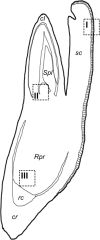
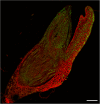
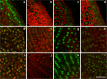
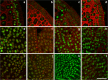
The transition of seeds from a dry to a metabolically active state requires significant changes in both the spatial and temporal patterns of gene expression, and this transcriptional reprogramming involves various modifications of the chromatin structure. There are several factors that can greatly influence the structure of chromatin, one of which is the chemical modifications of histone proteins and DNA itself. In this study, we analysed the distribution of three epigenetic markers, i.e. acetylation of histone H4 (H4K16ac) and histone H3 (H3K18ac) as well as DNA methylation (5mC) in Brachypodium distachyon embryos during the four stages of seed development-maturation, desiccation (quiescence), imbibition and germination. Our results indicate that both H4K16ac and H3K18ac are at a very high level in embryos during seed imbibition, but that the patterns of DNA methylation are considerably more stable in embryos during seed development.
Keywords: Brachypodium distachyon embryo; DNA methylation; Epigenetic modifications; H3K18ac; H4K16ac.
Figures
Similar articles
-
Spatial distribution of epigenetic modifications in Brachypodium distachyon embryos during seed maturation and germination.PLoS One. 2014 Jul 9;9(7):e101246. doi: 10.1371/journal.pone.0101246. eCollection 2014. PLoS One. 2014. PMID: 25006668 Free PMC article.
-
In situ analysis of epigenetic modifications in the chromatin of Brachypodium distachyon embryos.Plant Signal Behav. 2015;10(5):e1011948. doi: 10.1080/15592324.2015.1011948. Plant Signal Behav. 2015. PMID: 26039470 Free PMC article.
-
Allotetraploidization in Brachypodium May Have Led to the Dominance of One Parent's Metabolome in Germinating Seeds.Cells. 2021 Apr 7;10(4):828. doi: 10.3390/cells10040828. Cells. 2021. PMID: 33917018 Free PMC article.
-
Genes of the month: H3.3 histone genes: H3F3A and H3F3B.J Clin Pathol. 2021 Dec;74(12):753-758. doi: 10.1136/jclinpath-2021-207857. Epub 2021 Oct 19. J Clin Pathol. 2021. PMID: 34667098 Review.
-
Decoding histone 3 lysine methylation: Insights into seed germination and flowering.Curr Opin Plant Biol. 2024 Oct;81:102598. doi: 10.1016/j.pbi.2024.102598. Epub 2024 Jul 9. Curr Opin Plant Biol. 2024. PMID: 38986392 Review.
Cited by
-
Advances in Therapeutic Targeting of Cancer Stem Cells within the Tumor Microenvironment: An Updated Review.Cells. 2020 Aug 13;9(8):1896. doi: 10.3390/cells9081896. Cells. 2020. PMID: 32823711 Free PMC article. Review.
-
Germination and the Early Stages of Seedling Development in Brachypodium distachyon.Int J Mol Sci. 2018 Sep 25;19(10):2916. doi: 10.3390/ijms19102916. Int J Mol Sci. 2018. PMID: 30257527 Free PMC article.
-
Intertwined signatures of desiccation and drought tolerance in grasses.Proc Natl Acad Sci U S A. 2020 May 5;117(18):10079-10088. doi: 10.1073/pnas.2001928117. Epub 2020 Apr 23. Proc Natl Acad Sci U S A. 2020. PMID: 32327609 Free PMC article.
-
Thriving or Withering? Plant Molecular Cytogenetics in the First Quarter of the 21st Century.Int J Mol Sci. 2025 Jul 21;26(14):7013. doi: 10.3390/ijms26147013. Int J Mol Sci. 2025. PMID: 40725259 Free PMC article. Review.
-
Post-transcriptional regulation of seed dormancy and germination: Current understanding and future directions.Plant Commun. 2021 Feb 18;2(4):100169. doi: 10.1016/j.xplc.2021.100169. eCollection 2021 Jul 12. Plant Commun. 2021. PMID: 34327318 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

