Pharmacokinetics of Rytary®, An Extended-Release Capsule Formulation of Carbidopa-Levodopa
- PMID: 28236251
- PMCID: PMC5563351
- DOI: 10.1007/s40262-017-0511-y
Pharmacokinetics of Rytary®, An Extended-Release Capsule Formulation of Carbidopa-Levodopa
Abstract
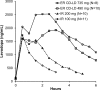
Parkinson's disease (PD) is a chronic progressive neurological disorder characterized by resting tremor, rigidity, bradykinesia, gait disturbance, and postural instability. Levodopa, the precursor to dopamine, coadministered with carbidopa or benserazide, aromatic amino acid decarboxylase inhibitors, is the most effective and widely used therapeutic agent in the treatment of PD. With continued levodopa treatment, a majority of patients develop motor complications such as dyskinesia and motor 'on-off' fluctuations, which are, in part, related to the fluctuations in plasma concentrations of levodopa. A new extended-release (ER) carbidopa-levodopa capsule product (also referred to as IPX066) was developed and approved in the US as Rytary® and in the EU as Numient®. The capsule formulation is designed to provide an initial rapid absorption of levodopa comparable to immediate-release (IR) carbidopa-levodopa, and to subsequently provide stable levodopa concentrations with reduced peak-to-trough excursions in plasma concentrations in order to reduce motor fluctuations associated with pulsatile stimulation of dopamine receptors and to minimize dyskinesia. Phase III studies of this ER carbidopa-levodopa capsule formulation in patients with PD have shown a significant reduction in 'off' time compared with IR carbidopa-levodopa and carbidopa-levodopa-entacapone. We present a review of the clinical pharmacokinetics and pharmacodynamics of this ER product of carbidopa-levodopa in healthy subjects and in patients with PD.
Keywords: Carbidopa; Entacapone; Levodopa; Levodopa Dose; Sinemet.
Figures



Similar articles
-
Carbidopa/Levodopa ER Capsules (Rytary(®), Numient™): A Review in Parkinson's Disease.CNS Drugs. 2016 Jan;30(1):79-90. doi: 10.1007/s40263-015-0306-3. CNS Drugs. 2016. PMID: 26692288 Review.
-
Single-Dose Pharmacokinetics and Pharmacodynamics of IPX203 in Patients With Advanced Parkinson Disease: A Comparison With Immediate-Release Carbidopa-Levodopa and With Extended-Release Carbidopa-Levodopa Capsules.Clin Neuropharmacol. 2019 Jan/Feb;42(1):4-8. doi: 10.1097/WNF.0000000000000314. Clin Neuropharmacol. 2019. PMID: 30520758 Free PMC article. Clinical Trial.
-
Comparison of the pharmacokinetics of an oral extended-release capsule formulation of carbidopa-levodopa (IPX066) with immediate-release carbidopa-levodopa (Sinemet(®)), sustained-release carbidopa-levodopa (Sinemet(®) CR), and carbidopa-levodopa-entacapone (Stalevo(®)).J Clin Pharmacol. 2015 Sep;55(9):995-1003. doi: 10.1002/jcph.514. Epub 2015 May 20. J Clin Pharmacol. 2015. PMID: 25855267 Free PMC article. Clinical Trial.
-
Safety of IPX066 , an extended release carbidopa-levodopa formulation, for the treatment of Parkinson's disease.Expert Opin Drug Saf. 2015 May;14(5):761-7. doi: 10.1517/14740338.2015.1015986. Epub 2015 Feb 19. Expert Opin Drug Saf. 2015. PMID: 25697185 Review.
-
Pharmacodynamics, Efficacy, and Safety of IPX203 in Parkinson Disease Patients With Motor Fluctuations.Clin Neuropharmacol. 2019 Sep/Oct;42(5):149-156. doi: 10.1097/WNF.0000000000000354. Clin Neuropharmacol. 2019. PMID: 31306216 Free PMC article. Clinical Trial.
Cited by
-
Differential Gait Decline in Parkinson's Disease Enhances Discrimination of Gait Freezers from Non-Freezers.J Parkinsons Dis. 2020;10(4):1657-1673. doi: 10.3233/JPD-201961. J Parkinsons Dis. 2020. PMID: 32925092 Free PMC article.
-
Contributions of Gut Bacteria and Diet to Drug Pharmacokinetics in the Treatment of Parkinson's Disease.Front Neurol. 2019 Oct 15;10:1087. doi: 10.3389/fneur.2019.01087. eCollection 2019. Front Neurol. 2019. PMID: 31681153 Free PMC article. Review.
-
Increased foot strike variability in Parkinson's disease patients with freezing of gait.Parkinsonism Relat Disord. 2018 Aug;53:58-63. doi: 10.1016/j.parkreldis.2018.04.032. Epub 2018 May 1. Parkinsonism Relat Disord. 2018. PMID: 29773512 Free PMC article.
-
Application of Pharmacokinetics and Pharmacodynamics in Product Life Cycle Management. A Case Study with a Carbidopa-Levodopa Extended-Release Formulation.AAPS J. 2017 May;19(3):607-618. doi: 10.1208/s12248-016-0032-x. Epub 2017 Jan 24. AAPS J. 2017. PMID: 28120254 Review.
-
Real-World Experience with Carbidopa-Levodopa Extended-Release Capsules (Rytary®): Results of a Nationwide Dose Conversion Survey.Parkinsons Dis. 2021 Feb 19;2021:6638088. doi: 10.1155/2021/6638088. eCollection 2021. Parkinsons Dis. 2021. PMID: 33688424 Free PMC article.
References
-
- Camargo SM, Vuille-dit-Bille RN, Mariotta L, Ramadan T, Huggel K, Singer D, Götze O, Verrey F. The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2014;351:114–123. doi: 10.1124/jpet.114.216317. - DOI - PubMed
-
- Chase TN, Mouradian MM, Engber TM. Motor response complications and the function of striatal efferent systems. Neurology. 1993;43(Suppl 6):S23–S27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

