Cardiovascular Effects of Stimulant and Non-Stimulant Medication for Children and Adolescents with ADHD: A Systematic Review and Meta-Analysis of Trials of Methylphenidate, Amphetamines and Atomoxetine
- PMID: 28236285
- PMCID: PMC5336546
- DOI: 10.1007/s40263-017-0410-7
Cardiovascular Effects of Stimulant and Non-Stimulant Medication for Children and Adolescents with ADHD: A Systematic Review and Meta-Analysis of Trials of Methylphenidate, Amphetamines and Atomoxetine
Abstract
Background: Many children and adolescents with attention deficit/hyperactivity disorder (ADHD) are treated with stimulant and non-stimulant medication. ADHD medication may be associated with cardiovascular effects. It is important to identify whether mean group effects translate into clinically relevant increases for some individual patients, and/or increase the risk for serious cardiovascular adverse events such as stroke or sudden death.
Objectives: To evaluate potential cardiovascular effects of these treatments, we conducted a systematic review and meta-analysis of the effects of methylphenidate (MPH), amphetamines (AMP), and atomoxetine (ATX) on diastolic and systolic blood pressure (DBP, SBP) and heart rate (HR) in children and adolescents with ADHD.
Methods: We conducted systematic searches in electronic databases (PsychINFO, EMBASE and Medline) to identify published trials which involved individuals who were (i) diagnosed with ADHD and were aged between 0-18 years; (ii) treated with MPH, AMP or ATX and (iii) had their DBP and SBP and/or HR measured at baseline (pre) and the endpoint (post) of the study treatment. Studies with an open-label design or a double-blind randomised control design of any duration were included. Statistical analysis involved calculating differences between pre- and post-treatment measurements for the various cardiovascular parameters divided by the pooled standard deviation. Further, we assessed the percentage of clinically relevant increased BP or HR, or documented arrhythmias.
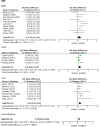
Results: Eighteen clinical trials met the inclusion criteria (10 for MPH, 5 for AMP, and 7 for ATX) with data from 5837 participants (80.7% boys) and average duration of 28.7 weeks (range 4-96 weeks). All three medications were associated with a small, but statistically significant pre-post increase of SBP (MPH: standard mean difference [SMD] 0.25, 95% confidence interval [CI] 0.08-0.42, p < 0.01; AMP: SMD 0.09, 95% CI 0.03-0.15, p < 0.01; ATX: SMD 0.16, 95% CI 0.04-0.27, p = 0.01). MPH did not have a pre-post effect on DBP and HR. AMP treatment was associated with a small but statistically significant pre-post increase of DBP (SMD 0.16, CI 0.03-0.29, p = 0.02), as was ATX treatment (SMD 0.22, CI 0.10-0.34, p < 0.01). AMP and ATX were associated with a small to medium statistically significant pre-post increase of HR (AMP: SMD 0.37, CI 0.13-0.60, p < 0.01; ATX: SMD 0.43, CI 0.26-0.60, p < 0.01). The head-to-head comparison of the three medications did not reveal significant differences. Sensitivity analyses revealed that AMP studies of <18 weeks reported higher effect sizes on DBP compared with longer duration studies (F(1) = 19.55, p = 0.05). Further, MPH studies published before 2007 reported higher effect sizes on SBP than studies after 2007 (F(1) = 5.346, p = 0.05). There was no effect of the following moderators: type of medication, doses, sample size, age, gender, type of ADHD, comorbidity or dropout rate. Participants on medication reported 737 (12.6%) other cardiovascular effects. Notably, 2% of patients discontinued their medication treatment due to any cardiovascular effect. However, in the majority of patients, the cardiovascular effects resolved spontaneously, medication doses were changed or the effects were not considered clinically relevant. There were no statistically significant differences between the medication treatments in terms of the severity of cardiovascular effects.
Conclusions: Statistically significant pre-post increases of SBP, DBP and HR were associated with AMP and ATX treatment in children and adolescents with ADHD, while MPH treatment had a statistically significant effect only on SBP in these patients. These increases may be clinically significant for a significant minority of individuals that experience larger increases. Since increased BP and HR in general are considered risk factors for cardiovascular morbidity and mortality during adult life, paediatric patients using ADHD medication should be monitored closely and regularly for HR and BP.
Conflict of interest statement
Conflict of interest
L. Hennissen and M.J. Bakker state no biomedical or financial interests or potential conflicts of interest.
T. Banaschewski has served in an advisory or consultancy role for Actelion, Hexal Pharma, Lilly, Medice, Novartis, Oxford Outcomes, PCM Scientific, Shire and Vifor Pharma. He has received conference support or speaker’s fees from Medice, Novartis and Shire. He is/has been involved in clinical trials conducted by Shire and Vifor Pharma. He received royalties from Hogrefe, Kohlhammer, CIP Medien and Oxford University Press. The present work is unrelated to the above grants and relationships.
S. Carucci collaborated within projects from the European Union (7th Framework Program), has received travel support from Shire Pharmaceutical Company and has collaborated as sub-investigator in clinical trials sponsored by Shire.
D. Coghill has received research funding from Shire and the European Union, he has received consulting fees from Shire, Novartis and Sandoz and payments for lectures from Shire, Eli Lilly, Janssen Cilag and Novartis. He has given expert testimony for GlaxoSmithKline and received royalties from Oxford University Press.
M. Danckaerts has been a member of the advisory boards of Shire and Neurotech Solutions and a speaker for Shire, Medici and Novartis (no product-related lectures) over the past 3 years. Within that period she received research grants from Janssen-Cilag, Shire, EU, FWO and KU Leuven.
R.W. Dittmann is a former employee of Eli Lilly & Co. and owner of Lilly stock. He has served in an advisory or consultancy role for Boehringer Ingelheim, Janssen-Cilag, Lilly, and Shire. He has received conference attendance support and received speaker’s fees from Boehringer Ingelheim, Lilly and Shire. He has been involved in clinical trials conducted by Ferring, Janssen-Cilag, Lilly, Lundbeck, Otsuka, Servier, Shire, and Sunovion. He has received research funding from the US National Institute of Mental Health (NIMH), the European Union (EU FP7), the German Research Foundation (DFG), the German Ministries of Research and Education (BMBF) and Health (BMG/BfArM), and the Volkswagen Foundation.
C. Hollis has received research grants from the European Union FP7 programme, H2020, National Institute of Health Research (NIHR) and Medical Research Council (MRC) during the conduct of the study; CH is a member of the European ADHD Guideline Group (EAGG) and NICE ADHD Guideline Committee. He is not an employee of any of these companies/research organisations.
H. Kovshoff states no biomedical or financial interests or potential conflicts of interest.
S. McCarthy has, in the past 3 years, received conference attendance and research support from Shire. She has received funding from the European Union (EU FP7).
P. Nagy has served in an advisory or consultancy role for Lilly, and has received conference attendance support from Shire. He has been involved in clinical trials conducted by Shire.
E. Rosenthal received funding from the EU FP7 grant and speakers fees from Shire.
E. Sonuga-Barke has been appointed visiting chairs at Ghent University, Aarhus University and University of Sussex. He has received speaker fees, consultancy, research funding and conference support from Shire Pharma, speaker fees from Janssen Cilag, consultancy from Neurotech solutions, Aarhus University, Copenhagen University and Berhanderling, Skolerne, Copenhagen, and KU Leuven; book royalties from OUP and Jessica Kingsley; grants awarded from MRC, ESRC, Wellcome Trust, Solent NHS Trust, European Union, Child Health Research Foundation New Zealand, NIHR, Nuffield Foundation, Fonds Wetenschappelijk Onderzoek-Vlaanderen (FWO) and MQ—Transforming Mental Health.
I.C. K. Wong received funding from the EU FP7 grant, Hong Kong Research Grant Council and Janssen Cilag for ADHD research. He is a member of UK NICE ADHD Guidelines Development Group.
A. Zuddas has been, in the last 3 years, a consultant to/member of advisory board of/and/or speaker for Angelini, Shire, Otsuka, Takeda and Ecu Farma. He has received research funding from Shire, Roche, Lundbeck, Vifor, FP7 (PERS, ADDUCE, Stop, Matrics) and the Sardinian Regional Government, and royalties from Oxford University Press and Giunti OS.
J. K. Buitelaar has been a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Bristol-Myer Squibb, Shering Plough, UCB, Shire, Lundbeck, Medice, Viforpharma, Novartis, Roche and Servier. He is not an employee of any of these companies, nor a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties.
Funding
The research leading to these results received support from the European Community’s Seventh Framework Programme (FP7/2007–2013) under Grant Agreement Number 260576 (ADDUCE). The Radboud University Medical Center paid the open access fee.
Figures



References
-
- Association, A.P. Diagnostic and statistical manual of mental disorders: DSM-5TM, 5th ed. Arlington: American Psychiatric Publishing, Inc; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

