Autologous lymphapheresis for the production of chimeric antigen receptor T cells
- PMID: 28236305
- PMCID: PMC5398918
- DOI: 10.1111/trf.14003
Autologous lymphapheresis for the production of chimeric antigen receptor T cells
Abstract
Background: The first step in manufacturing chimeric antigen receptor (CAR) T cells is to collect autologous CD3+ lymphocytes by apheresis. Patients, however, often have leukopenia or have other disease-related complications. We evaluated the feasibility of collecting adequate numbers of CD3+ cells, risk factors for inadequate collections, and the rate of adverse events.
Study design and methods: Apheresis lymphocyte collections from patients participating in three CAR T-cell clinical trials were reviewed. Collections were performed on the COBE Spectra by experienced nurses, with the goal of obtaining a minimum of 0.6 × 109 and a target of 2 × 109 CD3+ cells. Preapheresis peripheral blood counts, apheresis parameters, and product cell counts were analyzed.
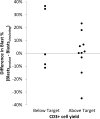
Results: Of the 71 collections, 69 (97%) achieved the minimum and 55 (77%) achieved the target. Before apheresis, the 16 patients with yields below the target had significantly lower proportions and absolute numbers of circulating lymphocytes and CD3+ lymphocytes and higher proportions of circulating blasts and NK cells than those who achieved the target (470 × 106 lymphocytes/L vs. 1340 × 106 lymphocytes/L, p = 0.008; 349 × 106 CD3+ cells/L vs. 914 × 106 CD3+ cells/L, p = 0.001; 17.6% blasts vs. 4.55% blasts, p = 0.029). Enrichment of blasts in the product compared to the peripheral blood occurred in four patients, including the two patients whose collections did not yield the minimum number of CD3+ cells. Apheresis complications occurred in 11 patients (15%) and, with one exception, were easily managed in the apheresis clinic.
Conclusions: In most patients undergoing CAR T-cell therapy, leukapheresis is well tolerated, and adequate numbers of CD3+ lymphocytes are collected.
Published 2017. This article is a U.S. Government work and is in the public domain in the USA.
Figures





Comment in
-
CAR-T cell manufacturing: time to put it in gear.Transfusion. 2017 May;57(5):1104-1106. doi: 10.1111/trf.14110. Transfusion. 2017. PMID: 28425600 No abstract available.
References
-
- Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev. 2015 - PubMed
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–28. - PMC - PubMed
-
- Brentjens RJ, Riviere I, Park JH, Davila ML, Wang X, Stefanski J, Taylor C, Yeh R, Bartido S, Borquez-Ojeda O, Olszewska M, Bernal Y, Pegram H, Przybylowski M, Hollyman D, Usachenko Y, Pirraglia D, Hosey J, Santos E, Halton E, Maslak P, Scheinberg D, Jurcic J, Heaney M, Heller G, Frattini M, Sadelain M. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–28. - PMC - PubMed
-
- Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, Borquez-Ojeda O, Qu J, Wasielewska T, He Q, Bernal Y, Rijo IV, Hedvat C, Kobos R, Curran K, Steinherz P, Jurcic J, Rosenblat T, Maslak P, Frattini M, Sadelain M. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

