Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial
- PMID: 28236467
- PMCID: PMC6168937
- DOI: 10.1016/S0140-6736(17)30396-3
Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial
Abstract
Background: Colorectal cancer is the third most common cancer worldwide. Previous analyses have only reported follow-up after flexible sigmoidoscopy for a maximum of 12 years. We aimed to examine colorectal cancer incidence and mortality after a single flexible sigmoidoscopy screening and 17 years of follow-up.
Methods: In this multicentre randomised trial (UK Flexible Sigmoidoscopy Screening Trial), done between Nov 14, 1994, and March 30, 1999, 170 432 eligible men and women, who had indicated on a previous questionnaire that they would probably attend screening if invited, were randomly assigned (1:2) to an intervention group (offered flexible sigmoidoscopy screening) or a control group (not contacted). Randomisation was done centrally in blocks of 12, and stratified by trial centre, general practice, and household type. The nature of the intervention did not allow the staff to be masked to arm of the trial; however, randomisation was done in batches so that the control group and participants not yet randomised were unaware of their allocation status. The primary outcomes were incidence and mortality of colorectal cancer. Hazard ratios (HRs) and 95% CIs for colorectal cancer incidence and mortality were estimated for intention-to-treat and per-protocol analyses. The trial is registered with ISRCTN, number 28352761.
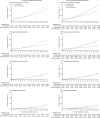
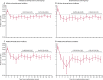
Findings: Our cohort analysis included 170 034 people: 112 936 in the control group and 57 098 in the intervention group, 40 621 (71%) of whom were screened and 16 477 (29%) were not screened. During screening and a median of 17·1 years' follow-up, colorectal cancer was diagnosed in 1230 individuals in the intervention group and 3253 in the control group, and 353 individuals in the intervention group versus 996 individuals in the control group died from colorectal cancer. In intention-to-treat analyses, colorectal cancer incidence was reduced by 26% (HR 0·74 [95% CI 0·70-0·80]; p<0·0001) in the intervention group versus the control group and colorectal cancer mortality was reduced by 30% (0·70 [0·62-0·79]; p<0·0001) in the intervention group versus the control group. In per-protocol analyses, adjusted for non-compliance, colorectal cancer incidence and mortality were 35% (HR 0·65 [95% CI 0·59-0·71]) and 41% (0·59 [0·49-0·70]) lower in the screened group.
Interpretation: A single flexible sigmoidoscopy continues to provide substantial protection from colorectal cancer diagnosis and death, with protection lasting at least 17 years.
Funding: National Institute for Health Research Efficacy and Mechanism Evaluation.
Copyright © 2017 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Flexible sigmoidoscopy screening: is once enough?Lancet. 2017 Apr 1;389(10076):1275-1277. doi: 10.1016/S0140-6736(17)30542-1. Epub 2017 Feb 22. Lancet. 2017. PMID: 28236465 No abstract available.
-
[Long-term results of colorectal cancer screening using sigmoidoscopy : UK flexible sigmoidoscopy screening trial (UKFSST)].Internist (Berl). 2017 Oct;58(10):1111-1113. doi: 10.1007/s00108-017-0309-x. Internist (Berl). 2017. PMID: 28812118 German. No abstract available.
-
Long-term effects of a flexible sigmoidoscopy screening after 17 years of follow up.Turk J Gastroenterol. 2017 Sep;28(5):427-428. doi: 10.5152/tjg.2017.020817. Turk J Gastroenterol. 2017. PMID: 28936974 No abstract available.
References
-
- Atkin WS, Cook CF, Cuzick J. Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentre randomised trial. Lancet. 2002;359:1291–1300. - PubMed
-
- Atkin WS, Edwards R, Kralj-Hans I. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. - PubMed
-
- Segnan N, Armaroli P, Bonelli L. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial-SCORE. J Natl Cancer Inst. 2011;103:1310–1322. - PubMed
-
- Mayor S. UK committee recommends flexible sigmoidoscopy to screen for bowel cancer. BMJ. 2011;342:d2325.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical