Novel Biomarkers of Human GM1 Gangliosidosis Reflect the Clinical Efficacy of Gene Therapy in a Feline Model
- PMID: 28236574
- PMCID: PMC5383552
- DOI: 10.1016/j.ymthe.2017.01.009
Novel Biomarkers of Human GM1 Gangliosidosis Reflect the Clinical Efficacy of Gene Therapy in a Feline Model
Abstract
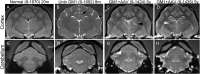
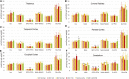
GM1 gangliosidosis is a fatal neurodegenerative disease that affects individuals of all ages. Favorable outcomes using adeno-associated viral (AAV) gene therapy in GM1 mice and cats have prompted consideration of human clinical trials, yet there remains a paucity of objective biomarkers to track disease status. We developed a panel of biomarkers using blood, urine, cerebrospinal fluid (CSF), electrodiagnostics, 7 T MRI, and magnetic resonance spectroscopy in GM1 cats-either untreated or AAV treated for more than 5 years-and compared them to markers in human GM1 patients where possible. Significant alterations were noted in CSF and blood of GM1 humans and cats, with partial or full normalization after gene therapy in cats. Gene therapy improved the rhythmic slowing of electroencephalograms (EEGs) in GM1 cats, a phenomenon present also in GM1 patients, but nonetheless the epileptiform activity persisted. After gene therapy, MR-based analyses revealed remarkable preservation of brain architecture and correction of brain metabolites associated with microgliosis, neuroaxonal loss, and demyelination. Therapeutic benefit of AAV gene therapy in GM1 cats, many of which maintain near-normal function >5 years post-treatment, supports the strong consideration of human clinical trials, for which the biomarkers described herein will be essential for outcome assessment.
Keywords: AAV gene therapy; gangliosidosis; lysosomal storage disorders; neurodegeneration.
Copyright © 2017 The American Society of Gene and Cell Therapy. All rights reserved.
Figures



References
-
- Regier D.S., Tifft C.J. GLB1-related disorders. In: Pagon R.A., editor. GeneReviews. University of Washington; 2013. pp. 1993–2016. - PubMed
-
- Meikle P.J., Hopwood J.J., Clague A.E., Carey W.F. Prevalence of lysosomal storage disorders. JAMA. 1999;281:249–254. - PubMed
-
- Cox T.M., Cachón-González M.B. The cellular pathology of lysosomal diseases. J. Pathol. 2012;226:241–254. - PubMed
-
- Okada S., O’Brien J.S. Generalized gangliosidosis: beta-galactosidase deficiency. Science. 1968;160:1002–1004. - PubMed
-
- Yoshiyuki Suzuki A.O., Nanba E., editors. Beta-galactosidase deficiency (beta-galactosidosis): GM1 gangliosidosis and Morquio B disease. McGraw-Hill; 2001. pp. 3775–3909.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

