Targeted Intraceptor Nanoparticle for Neovascular Macular Degeneration: Preclinical Dose Optimization and Toxicology Assessment
- PMID: 28236576
- PMCID: PMC5498805
- DOI: 10.1016/j.ymthe.2017.01.014
Targeted Intraceptor Nanoparticle for Neovascular Macular Degeneration: Preclinical Dose Optimization and Toxicology Assessment
Abstract
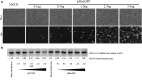
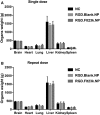
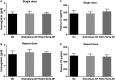
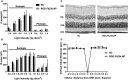
Neovascular age-related macular degeneration (AMD) is treated with anti-VEGF intravitreal injections, which can cause geographic atrophy, infection, and retinal fibrosis. To minimize these toxicities, we developed a nanoparticle delivery system for recombinant Flt23k intraceptor plasmid (RGD.Flt23k.NP) to suppress VEGF intracellularly within choroidal neovascular (CNV) lesions in a laser-induced CNV mouse model through intravenous administration. In the current study, we examined the efficacy and safety of RGD.Flt23k.NP in mice. The effect of various doses was determined using fluorescein angiography and optical coherence tomography to evaluate CNV leakage and volume. Efficacy was determined by the rate of inhibition of CNV volume at 2 weeks post-treatment. RGD.Flt23k.NP had peak efficacy at a dose range of 30-60 μg pFlt23k/mouse. Using the lower dose (30 μg pFlt23k/mouse), RGD.Flt23k.NP safety was determined both in single-dose groups and in repeat-dose (three times) groups by measuring body weight, organ weight, hemoglobin levels, complement C3 levels, and histological changes in vital organs. Neither toxicity nor inflammation from RGD.Flt23k.NP was detected. No side effect was detected on visual function. Thus, systemic RGD.Flt23k.NP may be an alternative to standard intravitreal anti-VEGF therapy for the treatment of neovascular AMD.
Keywords: RGD.Flt23k.NP efficacy; RGD.Flt23k.NP safety; age-related macular degeneration.
Copyright © 2017. Published by Elsevier Inc.
Figures




References
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. - PubMed
-
- Klein R., Chou C.F., Klein B.E., Zhang X., Meuer S.M., Saaddine J.B. Prevalence of age-related macular degeneration in the US population. Arch. Ophthalmol. 2011;129:75–80. - PubMed
-
- Wong T.Y., Chakravarthy U., Klein R., Mitchell P., Zlateva G., Buggage R., Fahrbach K., Probst C., Sledge I. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–126. - PubMed
-
- Klein R., Klein B.E., Tomany S.C., Meuer S.M., Huang G.H. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–1779. - PubMed
-
- van der Reis M.I., La Heij E.C., De Jong-Hesse Y., Ringens P.J., Hendrikse F., Schouten J.S. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31:1449–1469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

