Catalytic competence, structure and stability of the cancer-associated R139W variant of the human NAD(P)H:quinone oxidoreductase 1 (NQO1)
- PMID: 28236663
- PMCID: PMC6250432
- DOI: 10.1111/febs.14051
Catalytic competence, structure and stability of the cancer-associated R139W variant of the human NAD(P)H:quinone oxidoreductase 1 (NQO1)
Abstract
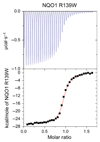
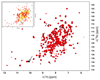
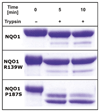
The human NAD(P)H:quinone oxidoreductase 1 (NQO1; EC1.6.99.2) is an essential enzyme in the antioxidant defence system. Furthermore, NQO1 protects tumour suppressors like p53, p33ING1b and p73 from proteasomal degradation. The activity of NQO1 is also exploited in chemotherapy for the activation of quinone-based treatments. Various single nucleotide polymorphisms are known, such as NQO1*2 and NQO1*3 yielding protein variants of NQO1 with single amino acid replacements, i.e. P187S and R139W, respectively. While the former NOQ1 variant is linked to a higher risk for specific kinds of cancer, the role, if any, of the arginine 139 to tryptophan exchange in disease development remains obscure. On the other hand, mitomycin C-resistant human colon cancer cells were shown to harbour the NQO1*3 variant resulting in substantially reduced enzymatic activity. However, the molecular cause for this decrease remains unclear. In order to resolve this issue, recombinant NQO1 R139W has been characterized biochemically and structurally. In this report, we show by X-ray crystallography and 2D-NMR spectroscopy that this variant adopts the same structure both in the crystal as well as in solution. Furthermore, the kinetic parameters obtained for the variant are similar to those reported for the wild-type protein. Similarly, thermostability of the variant was only slightly affected by the amino acid replacement. Therefore, we conclude that the previously reported effects in human cancer cells cannot be attributed to protein stability or enzyme activity. Instead, it appears that loss of exon 4 during maturation of a large fraction of pre-mRNA is the major reason of the observed lack of enzyme activity and hence reduced activation of quinone-based chemotherapeutics.
Keywords: NMR-spectroscopy; cancer; crystal structure; enzyme kinetics; microcalorimetry; single nucleotide polymorphism.
© 2017 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Figures





References
-
- Ross D, Siegel D. NAD(P)H:quinone oxidoreductase 1 (NQO1, DT-diaphorase), functions and pharmacogenetics. Methods Enzymol. 2004;382:115–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

