Test-treatment RCTs are susceptible to bias: a review of the methodological quality of randomized trials that evaluate diagnostic tests
- PMID: 28236806
- PMCID: PMC5326492
- DOI: 10.1186/s12874-016-0287-z
Test-treatment RCTs are susceptible to bias: a review of the methodological quality of randomized trials that evaluate diagnostic tests
Abstract
Background: There is a growing recognition for the need to expand our evidence base for the clinical effectiveness of diagnostic tests. Many international bodies are calling for diagnostic randomized controlled trials to provide the most rigorous evidence of impact to patient health. Although these so-called test-treatment RCTs are very challenging to undertake due to their methodological complexity, they have not been subjected to a systematic appraisal of their methodological quality. The extent to which these trials may be producing biased results therefore remains unknown. We set out to address this issue by conducting a methodological review of published test-treatment trials to determine how often they implement adequate methods to limit bias and safeguard the validity of results.
Methods: We ascertained all test-treatment RCTs published 2004-2007, indexed in CENTRAL, including RCTs which randomized patients to diagnostic tests and measured patient outcomes after treatment. Tests used for screening, monitoring or prognosis were excluded. We assessed adequacy of sequence generation, allocation concealment and intention-to-treat, appropriateness of primary analyses, blinding and reporting of power calculations, and extracted study characteristics including the primary outcome.
Results: One hundred three trials compared 105 control with 119 experimental interventions, and reported 150 primary outcomes. Randomization and allocation concealment were adequate in 57 and 37% of trials. Blinding was uncommon (patients 5%, clinicians 4%, outcome assessors 21%), as was an adequate intention-to-treat analysis (29%). Overall 101 of 103 trials (98%) were at risk of bias, as judged using standard Cochrane criteria.
Conclusion: Test-treatment trials are particularly susceptible to attrition and inadequate primary analyses, lack of blinding and under-powering. These weaknesses pose much greater methodological and practical challenges to conducting reliable RCT evaluations of test-treatment strategies than standard treatment interventions. We suggest a cautious approach that first examines whether a test-treatment intervention can accommodate the methodological safeguards necessary to minimize bias, and highlight that test-treatment RCTs require different methods to ensure reliability than standard treatment trials. Please see the companion paper to this article: http://bmcmedresmethodol.biomedcentral.com/articles/10.1186/s12874-016-0286-0 .
Keywords: Bias; Diagnostic accuracy; Methodological quality; Patient outcomes; RCT; Test evaluation; Test-treatment.
Figures
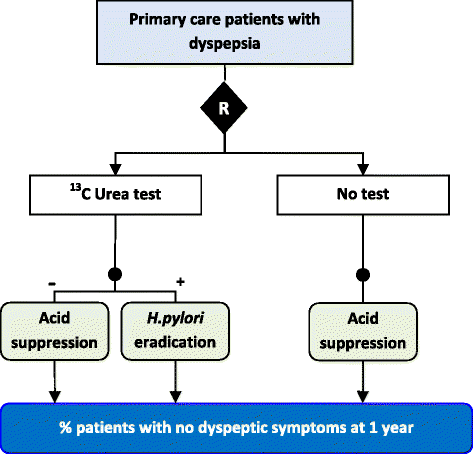
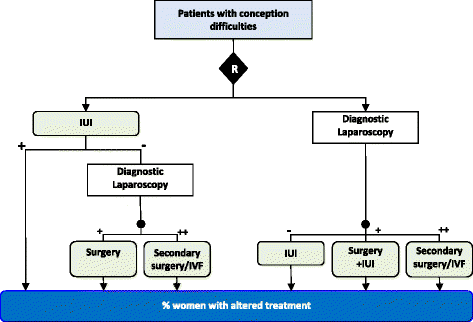
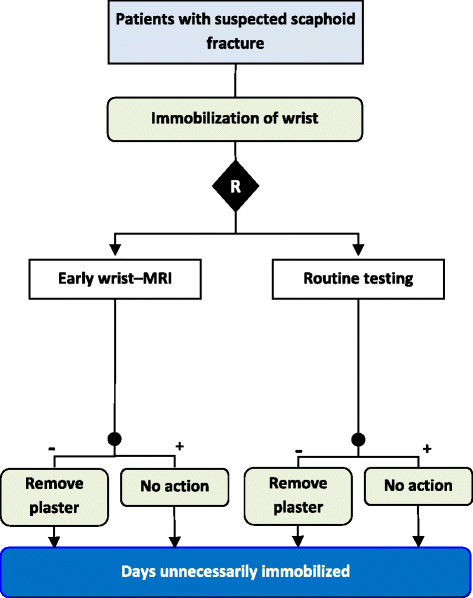
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

