Regulation of Cell Polarity by PAR-1/MARK Kinase
- PMID: 28236972
- PMCID: PMC5943083
- DOI: 10.1016/bs.ctdb.2016.11.001
Regulation of Cell Polarity by PAR-1/MARK Kinase
Abstract
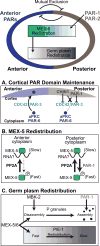
PAR-1/MARK kinases are conserved serine/threonine kinases that are essential regulators of cell polarity. PAR-1/MARK kinases localize and function in opposition to the anterior PAR proteins to control the asymmetric distribution of factors in a wide variety polarized cells. In this review, we discuss the mechanisms that control the localization and activity of PAR-1/MARK kinases, including their antagonistic interactions with the anterior PAR proteins. We focus on the role PAR-1 plays in the asymmetric division of the Caenorhabditis elegans zygote, in the establishment of the anterior/posterior axis in the Drosophila oocyte and in the control of microtubule dynamics in mammalian neurons. In addition to conserved aspects of PAR-1 biology, we highlight the unique ways in which PAR-1 acts in these distinct cell types to orchestrate their polarization. Finally, we review the connections between disruptions in PAR-1/MARK function and Alzheimer's disease and cancer.
Keywords: C. elegans; Cell polarity; Drosophila; MARK; Microtubule-associated protein; PAR proteins; PAR-1.
© 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Beghini A, Magnani I, Roversi G, Piepoli T, Di Terlizzi S, Moroni RF, Pollo B, Fuhrman Conti AM, Cowell JK, Finocchiaro G, Larizza L. The neural progenitor-restricted isoform of the MARK4 gene in 19q13.2 is upregulated in human gliomas and overexpressed in a subset of glioblastoma cell lines. Oncogene. 2003;22:2581–2591. - PubMed
-
- Benton R, Palacios IM, Johnston DS. Drosophila 14-3-3/PAR-5 Is an Essential Mediator of PAR-1 Function in Axis Formation. Developmental Cell. 2002;3:659–671. - PubMed
-
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

