Identification and characterization of glycation adducts on osteocalcin
- PMID: 28237256
- PMCID: PMC5490980
- DOI: 10.1016/j.ab.2017.02.011
Identification and characterization of glycation adducts on osteocalcin
Abstract
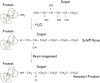
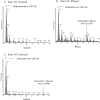
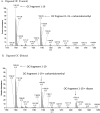
Osteocalcin is an important extracellular matrix bone protein that contributes to the structural properties of bone through its interactions with hydroxyapatite mineral and with collagen I. This role may be affected by glycation, a labile modification the levels of which has been shown to correlate with bone fragility. Glycation starts with the spontaneous addition of a sugar onto a free amine group on a protein, forming an Amadori product, and then proceeds through several environment-dependent stages resulting in the formation of an advanced glycation end product. Here, we induce the first step of this modification on synthetic osteocalcin, and then use multiple mass spectrometry fragmentation techniques to determine the location of this modification. Collision-induced dissociation resulted in spectra dominated by neutral loss, and was unable to identify Amadori products. Electron-transfer dissociation showed that the Amadori product formed solely on osteocalcin's N-terminus. This suggests that the glycation of osteocalcin is unlikely to interfere with osteocalcin's interaction with hydroxyapatite. Instead, glycation may interfere with its interaction with collagen I or another bone protein, osteopontin. Potentially, the levels of glycated osteocalcin fragments released from bone during bone resorption could be used to assess bone quality, should the N-terminal fragments be targeted.
Keywords: Bone; Bone matrix; Glycation; Non-collagenous protein; Osteocalcin.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

References
-
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg CM, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature Letters. 1996;382:448–452. - PubMed
-
- Hauschka PV, Carr SA. Calcium-Dependent a-Helical Structure in Osteocalcin. Biochemistry. 1982;21:2538–2547. - PubMed
-
- Boskey AL, Gadaleta S, Gundberg CM, Doty SB, Ducy P, Karsenty G. Fourier Transform Infrared Microspectroscopic Analysis of Bones of Osteocalcin-Deficient Mice Provides Insight Into the Function of Osteocalcin. Bone. 1998;23:187–196. - PubMed
-
- Poundarik AA, Gundberg CM, Vashishth D. Non-collageneous proteins influence bone crystal size and morphology: A SAXS study. IEEE. 2011
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

