African Genetic Diversity: Implications for Cytochrome P450-mediated Drug Metabolism and Drug Development
- PMID: 28237373
- PMCID: PMC5360579
- DOI: 10.1016/j.ebiom.2017.02.017
African Genetic Diversity: Implications for Cytochrome P450-mediated Drug Metabolism and Drug Development
Abstract
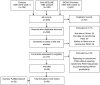
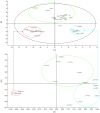
Genetic diversity is greater in Africa than in other continental populations. Genetic variability in genes encoding drug metabolizing enzymes may contribute to the high numbers of adverse drug reactions reported in Africa. We reviewed publications (1995-April 2016) reporting frequencies of known cytochrome P450 (CYP) variants in African populations. Using principal components analysis (PCA) we identified CYP alleles of potential clinical relevance with a marked difference in distribution in Africa, compared with Asian and Caucasian populations. These were CYP2B6*6, CYP2C8*2, CYP2D6*3, CYP2D6*17, CYP2D6*29, CYP3A5*6, and CYP3A5*7. We show clearly that there is greater diversity in CYP distribution in Africa than in other continental populations and identify a need for optimization of drug therapy and drug development there. Further pharmacogenetic studies are required to confirm the CYP distributions we identified using PCA, to discover uniquely African alleles and to identify populations at a potentially increased risk of drug-induced adverse events or drug inefficacy.
Keywords: Africa; CYP450; Drug development; Drug metabolism; Genetic diversity; Pharmacogenetics.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
References
-
- Alessandrini M., Pepper M.S. Priority pharmacogenetics for the African continent: focus on CYP450. Pharmacogenomics. 2014;15:385–400. - PubMed
-
- Ampadu H.H., Hoekman J., De Bruin M.L., Pal S.N., Olsson S., Sartori D., Leufkens H.G., Dodoo A.N. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf. 2016;39:335–345. - PMC - PubMed
-
- Bell G.C., Caudle K.E., Whirl-Carillo M., Gordon R.J., Hikino K., Prows C.A., Gaedigk A., Agundez J.A., Sadhasivam S., Klein T.E., Schwab M. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for the CYP2D6 genotype and use of ondansetron and tropisetron. Clin. Pharmacol. Ther. 2016 (Epub ahead of print) - PMC - PubMed
-
- Birdwell K.A., Decker B., Barbarino J.M., Peterson J.F., Stein C.M., Sadee W., Wang D., Vinks A.A., He Y., Swen J.J., Leeder J.S., Van Schaik R., Thummel K.E., Klein T.E., Caudle K.E., Macphee I.A. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 genotype and tacrolimus dosing. Clin. Pharmacol. Ther. 2015;98 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

