AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: Implications toward suppression of neointimal hyperplasia
- PMID: 28237515
- PMCID: PMC5392421
- DOI: 10.1016/j.phrs.2017.02.016
AdipoRon, an adiponectin receptor agonist, attenuates PDGF-induced VSMC proliferation through inhibition of mTOR signaling independent of AMPK: Implications toward suppression of neointimal hyperplasia
Abstract
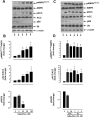
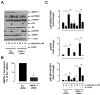
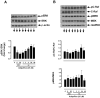
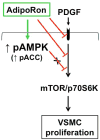
Hypoadiponectinemia is associated with an increased risk of coronary artery disease. Although adiponectin replenishment mitigates neointimal hyperplasia and atherosclerosis in mouse models, adiponectin therapy has been hampered in a clinical setting due to its large molecular size. Recent studies demonstrate that AdipoRon (a small-molecule adiponectin receptor agonist) improves glycemic control in type 2 diabetic mice and attenuates postischemic cardiac injury in adiponectin-deficient mice, in part, through activation of AMP-activated protein kinase (AMPK). To date, it remains unknown as to whether AdipoRon regulates vascular smooth muscle cell (VSMC) proliferation, which plays a major role in neointima formation. In the present study, oral administration of AdipoRon (50mg/kg) in C57BL/6J mice significantly diminished arterial injury-induced neointima formation by ∼57%. Under in vitro conditions, AdipoRon treatment led to significant inhibition of platelet-derived growth factor (PDGF)-induced VSMC proliferation, DNA synthesis, and cyclin D1 expression. While AdipoRon induced a rapid and sustained activation of AMPK, it also diminished basal and PDGF-induced phosphorylation of mTOR and its downstream targets, including p70S6K/S6 and 4E-BP1. However, siRNA-mediated AMPK downregulation showed persistent inhibition of p70S6K/S6 and 4E-BP1 phosphorylation, indicating AMPK-independent effects for AdipoRon inhibition of mTOR signaling. In addition, AdipoRon treatment resulted in a sustained and transient decrease in PDGF-induced phosphorylation of Akt and ERK, respectively. Furthermore, PDGF receptor-β tyrosine phosphorylation, which controls the phosphorylation state of Akt and ERK, was diminished upon AdipoRon treatment. Together, the present findings suggest that orally-administered AdipoRon has the potential to limit restenosis after angioplasty by targeting mTOR signaling independent of AMPK activation.
Keywords: AMPK; AdipoRon; Arterial injury; PDGF; Vascular smooth muscle cells; mTOR.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures











Similar articles
-
Pioglitazone, a PPARγ agonist, attenuates PDGF-induced vascular smooth muscle cell proliferation through AMPK-dependent and AMPK-independent inhibition of mTOR/p70S6K and ERK signaling.Biochem Pharmacol. 2016 Feb 1;101:54-70. doi: 10.1016/j.bcp.2015.11.026. Epub 2015 Nov 28. Biochem Pharmacol. 2016. PMID: 26643070 Free PMC article.
-
Sulforaphane inhibits platelet-derived growth factor-induced vascular smooth muscle cell proliferation by targeting mTOR/p70S6kinase signaling independent of Nrf2 activation.Pharmacol Res. 2017 May;119:251-264. doi: 10.1016/j.phrs.2017.02.010. Epub 2017 Feb 14. Pharmacol Res. 2017. PMID: 28212891 Free PMC article.
-
Activation of AMPK by Telmisartan Decreases Basal and PDGF-stimulated VSMC Proliferation via Inhibiting the mTOR/p70S6K Signaling Axis.J Korean Med Sci. 2020 Sep 7;35(35):e289. doi: 10.3346/jkms.2020.35.e289. J Korean Med Sci. 2020. PMID: 32893519 Free PMC article.
-
Mechanisms of Adiponectin Action: Implication of Adiponectin Receptor Agonism in Diabetic Kidney Disease.Int J Mol Sci. 2019 Apr 10;20(7):1782. doi: 10.3390/ijms20071782. Int J Mol Sci. 2019. PMID: 30974901 Free PMC article. Review.
-
Exploring the Logic and Conducting a Comprehensive Evaluation of the Adiponectin Receptor Agonists AdipoRon and AdipoAI's Impacts on Bone Metabolism and Repair-A Systematic Review.Curr Med Chem. 2025;32(6):1168-1194. doi: 10.2174/0109298673308301240821052742. Curr Med Chem. 2025. PMID: 39206478
Cited by
-
AdipoRon Treatment Induces a Dose-Dependent Response in Adult Hippocampal Neurogenesis.Int J Mol Sci. 2021 Feb 19;22(4):2068. doi: 10.3390/ijms22042068. Int J Mol Sci. 2021. PMID: 33669795 Free PMC article.
-
Corylin Inhibits Vascular Cell Inflammation, Proliferation and Migration and Reduces Atherosclerosis in ApoE-Deficient Mice.Antioxidants (Basel). 2020 Mar 25;9(4):275. doi: 10.3390/antiox9040275. Antioxidants (Basel). 2020. PMID: 32218307 Free PMC article.
-
AdipoRon Alleviates Free Fatty Acid-Induced Myocardial Cell Injury Via Suppressing Nlrp3 Inflammasome Activation.Diabetes Metab Syndr Obes. 2019 Oct 23;12:2165-2179. doi: 10.2147/DMSO.S221841. eCollection 2019. Diabetes Metab Syndr Obes. 2019. PMID: 31749627 Free PMC article.
-
AKT Mediates Adiponectin-Dependent Regulation of VSMC Phenotype.Cells. 2023 Oct 20;12(20):2493. doi: 10.3390/cells12202493. Cells. 2023. PMID: 37887338 Free PMC article.
-
Bushenkangshuai Tablet Reduces Atherosclerotic Lesion by Improving Blood Lipids Metabolism and Inhibiting Inflammatory Response via TLR4 and NF-κB Signaling Pathway.Evid Based Complement Alternat Med. 2018 Jan 24;2018:1758383. doi: 10.1155/2018/1758383. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 29619063 Free PMC article.
References
-
- Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. - PubMed
-
- Otsuka F, Sugiyama S, Kojima S, Maruyoshi H, Funahashi T, Matsui K, Sakamoto T, Yoshimura M, Kimura K, Umemura S, Ogawa H. Plasma adiponectin levels are associated with coronary lesion complexity in men with coronary artery disease. J Am Coll Cardiol. 2006;48:1155–1162. - PubMed
-
- von Eynatten M, Humpert PM, Bluemm A, Lepper PM, Hamann A, Allolio B, Nawroth PP, Bierhaus A, Dugi KA. High-molecular weight adiponectin is independently associated with the extent of coronary artery disease in men. Atherosclerosis. 2008;199:123–128. - PubMed
-
- Kitta Y, Takano H, Nakamura T, Kodama Y, Umetani K, Fujioka D, Saito Y, Kawabata K, Obata JE, Mende A, Kobayashi T, Kugiyama K. Low adiponectin levels predict late in-stent restenosis after bare metal stenting in native coronary arteries. Int J Cardiol. 2008;131:78–82. - PubMed
-
- Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

