A Drosophila model of myeloproliferative neoplasm reveals a feed-forward loop in the JAK pathway mediated by p38 MAPK signalling
- PMID: 28237966
- PMCID: PMC5399568
- DOI: 10.1242/dmm.028118
A Drosophila model of myeloproliferative neoplasm reveals a feed-forward loop in the JAK pathway mediated by p38 MAPK signalling
Abstract
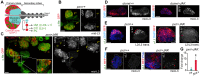
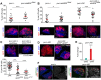
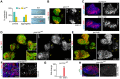
Myeloproliferative neoplasms (MPNs) of the Philadelphia-negative class comprise polycythaemia vera, essential thrombocythaemia and primary myelofibrosis (PMF). They are associated with aberrant numbers of myeloid lineage cells in the blood, and in the case of overt PMF, with development of myelofibrosis in the bone marrow and failure to produce normal blood cells. These diseases are usually caused by gain-of-function mutations in the kinase JAK2. Here, we use Drosophila to investigate the consequences of activation of the JAK2 orthologue in haematopoiesis. We have identified maturing haemocytes in the lymph gland, the major haematopoietic organ in the fly, as the cell population susceptible to induce hypertrophy upon targeted overexpression of JAK. We show that JAK activates a feed-forward loop, including the cytokine-like ligand Upd3 and its receptor, Domeless, which are required to induce lymph gland hypertrophy. Moreover, we present evidence that p38 MAPK signalling plays a key role in this process by inducing expression of the ligand Upd3. Interestingly, we also show that forced activation of the p38 MAPK pathway in maturing haemocytes suffices to generate hypertrophic organs and the appearance of melanotic tumours. Our results illustrate a novel pro-tumourigenic crosstalk between the p38 MAPK pathway and JAK signalling in a Drosophila model of MPNs. Based on the shared molecular mechanisms underlying MPNs in flies and humans, the interplay between Drosophila JAK and p38 signalling pathways unravelled in this work might have translational relevance for human MPNs.
Keywords: Drosophila; Haemocyte; Hypertrophy; JAK; Myeloproliferative neoplasm; p38 MAPK.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures

Similar articles
-
Gα73B is a downstream effector of JAK/STAT signalling and a regulator of Rho1 in Drosophila haematopoiesis.J Cell Sci. 2014 Jan 1;127(Pt 1):101-10. doi: 10.1242/jcs.132852. Epub 2013 Oct 25. J Cell Sci. 2014. PMID: 24163435
-
A Genetic Screen Reveals an Unexpected Role for Yorkie Signaling in JAK/STAT-Dependent Hematopoietic Malignancies in Drosophila melanogaster.G3 (Bethesda). 2017 Aug 7;7(8):2427-2438. doi: 10.1534/g3.117.044172. G3 (Bethesda). 2017. PMID: 28620086 Free PMC article.
-
Macrophage-like Blood Cells Are Involved in Inter-Tissue Communication to Activate JAK/STAT Signaling, Inducing Antitumor Turandot Proteins in Drosophila Fat Body via the TNF-JNK Pathway.Int J Mol Sci. 2024 Dec 6;25(23):13110. doi: 10.3390/ijms252313110. Int J Mol Sci. 2024. PMID: 39684820 Free PMC article.
-
Mouse models of myeloproliferative neoplasms: JAK of all grades.Dis Model Mech. 2011 May;4(3):311-7. doi: 10.1242/dmm.006817. Dis Model Mech. 2011. PMID: 21558064 Free PMC article. Review.
-
[Biological functions of Jak/Stat signaling pathway in Drosophila].Genetika. 2013 Nov;49(11):1245-50. Genetika. 2013. PMID: 25470924 Review. Russian.
Cited by
-
Autophagy controls differentiation of Drosophila blood cells by regulating Notch levels in response to nutrient availability.Nat Commun. 2025 Jul 1;16(1):5858. doi: 10.1038/s41467-025-58389-y. Nat Commun. 2025. PMID: 40595449 Free PMC article.
-
Regulation of Drosophila Hematopoiesis in Lymph Gland: From a Developmental Signaling Point of View.Int J Mol Sci. 2020 Jul 24;21(15):5246. doi: 10.3390/ijms21155246. Int J Mol Sci. 2020. PMID: 32722007 Free PMC article. Review.
-
GDF11 induces differentiation and apoptosis and inhibits migration of C17.2 neural stem cells via modulating MAPK signaling pathway.PeerJ. 2018 Sep 4;6:e5524. doi: 10.7717/peerj.5524. eCollection 2018. PeerJ. 2018. PMID: 30202652 Free PMC article.
-
Rab5 and Rab11 maintain hematopoietic homeostasis by restricting multiple signaling pathways in Drosophila.Elife. 2021 Feb 9;10:e60870. doi: 10.7554/eLife.60870. Elife. 2021. PMID: 33560224 Free PMC article.
-
Drosophila Innate Immunity Involves Multiple Signaling Pathways and Coordinated Communication Between Different Tissues.Front Immunol. 2022 Jul 7;13:905370. doi: 10.3389/fimmu.2022.905370. eCollection 2022. Front Immunol. 2022. PMID: 35911716 Free PMC article. Review.
References
-
- Adachi-Yamada T., Nakamura M., Irie K., Tomoyasu Y., Sano Y., Mori E., Goto S., Ueno N., Nishida Y. and Matsumoto K. (1999). p38 mitogen-activated protein kinase can be involved in transforming growth factor β superfamily signal transduction in drosophila wing morphogenesis. Mol. Cell. Biol. 19, 2322-2329. 10.1128/MCB.19.3.2322 - DOI - PMC - PubMed
-
- Anderl I., Vesala L., Ihalainen T. O., Vanha-Aho L.-M., Andó I., Rämet M. and Hultmark D. (2016). Transdifferentiation and proliferation in two distinct hemocyte lineages in Drosophila melanogaster larvae after wasp infection. PLoS Pathog. 12, e1005746 10.1371/journal.ppat.1005746 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

