Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients
- PMID: 28238223
- PMCID: PMC6464265
- DOI: 10.1002/14651858.CD009966.pub2
Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients
Update in
-
Glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients.Cochrane Database Syst Rev. 2020 Jul 30;8(8):CD009966. doi: 10.1002/14651858.CD009966.pub3. Cochrane Database Syst Rev. 2020. PMID: 32803882 Free PMC article.
Abstract
Background: Kidney transplantation is the preferred form of kidney replacement therapy for patients with end-stage kidney disease (ESKD) and is often complicated by worsening or new-onset diabetes. Management of hyperglycaemia is important to reduce post-transplant and diabetes-related complications. The safety and efficacy of glucose-lowering agents after kidney transplantation is largely unknown.
Objectives: To evaluate the efficacy and safety of pharmacological interventions for lowering glucose levels in patients who have undergone kidney transplantation and have diabetes.
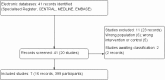
Search methods: We searched the Cochrane Kidney and Transplant Specialised Register to 15 April 2016 through contact with the Information Specialist using search terms relevant to this review. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE, and EMBASE; handsearching conference proceedings; and searching the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria: All randomised controlled trials (RCTs), quasi-RCTs and cross-over studies examining head-to-head comparisons of active regimens of glucose-lowering therapy or active regimen compared with placebo/standard care in patients who have received a kidney transplant and have diabetes were eligible for inclusion.
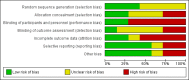
Data collection and analysis: Two authors independently assessed study eligibility and quality and performed data extraction. Continuous outcomes were expressed as post-treatment mean differences (MD) or standardised mean difference (SMD). Adverse events were expressed as post-treatment absolute risk differences (RD). Dichotomous clinical outcomes were presented as risk ratios (RR) with 95% confidence intervals (CI).
Main results: We included seven studies that involved a total of 399 kidney transplant recipients. All included studies had observed heterogeneity in the patient population, interventions and measured outcomes or missing data (which was unavailable despite correspondence with authors). Many studies had incompletely reported methodology preventing meta-analysis and leading to low confidence in treatment estimates.Three studies with 241 kidney transplant recipients examined the use of more intensive compared to less intensive insulin therapy in kidney transplant recipients with pre-existing type 1 or 2 diabetes. Evidence for the effects of more intensive compared to less intensive insulin therapy on transplant graft survival, HbA1c, fasting blood glucose, all cause mortality and adverse effects including hypoglycaemia was of very low quality. More intensive versus less intensive insulin therapy resulted in no difference in transplant or graft survival over three to five years in one study while another study showed that more intensive versus less intensive insulin therapy resulted in more rejection events over the three year follow-up (11 events in total; 9 in the more intensive group, P = 0.01). One study showed that more intensive insulin therapy resulted in a lower mean HbA1c (10 ± 0.8% versus 13 ± 0.9%) and lower fasting blood glucose (7.22 ± 0.5 mmol/L versus 13.44 ± 1.22 mmol/L) at 13 months compared with standard insulin therapy. Another study showed no difference between more intensive compared to less intensive insulin therapy on all-cause mortality over a five year follow-up period. All studies showed either an increased frequency of hypoglycaemia or severe hypoglycaemia episodes.Three studies with a total of 115 transplant recipients examined the use of DPP4 inhibitors for new-onset diabetes after transplantation. Evidence for the treatment effect of DPP4 inhibitors on transplant or graft survival, HbA1c and fasting blood glucose levels, all cause mortality, and adverse events including hypoglycaemia was of low quality. One study comparing vildagliptin to placebo and another comparing sitagliptin to placebo showed no difference in transplant or graft survival over two to four months of follow-up. One study comparing vildagliptin to placebo showed no significant change in estimated glomerular filtration rate from baseline (1.9 ± 10.3 mL/min/1.73 m2, P = 0.48 and 2.1 ± 6.1 mL/min/1.73 m2, P = 0.22) and no deaths, in either treatment group over three months of follow-up. One study comparing vildagliptin to placebo showed a lower HbA1c level (mean ± SD) (6.3 ± 0.5% versus versus 6.7 ± 0.6%, P = 0.03) and trend towards a greater lowering of fasting blood glucose (-0.91 ± -0.92 mmol/L versus vs -0.19 ± 1.16 mmol/L, P = 0.08) with vildagliptin. One study comparing sitagliptin to insulin glargine showed an equivalent lowering of HbA1c (-0.6 ± 0.5% versus -0.6 ± 0.6%, P = NS) and fasting blood glucose (4.92 ± 1.42 versus 4.76 ± 1.09 mmol/L, P = NS) with sitagliptin. For the outcome of hypoglycaemia, one study comparing vildagliptin to placebo reported no episodes of hypoglycaemia, one study comparing sitagliptin to insulin glargine reported fewer episodes of hypoglycaemia with sitagliptin (3/28 patients; 10.7% versus 5/28; 17.9%) and one cross-over study of sitagliptin and placebo reported two episodes of asymptomatic moderate hypoglycaemia (2 to 3.9 mmol/L) when sitagliptin was administered with glipizide. All three studies reported no drug interactions between DPP4 inhibitors and the immunosuppressive agents taken.Evidence for the treatment effect of pioglitazone for treating pre-existing diabetes was of low quality. One study with 62 transplant recipients compared the use of pioglitazone with insulin to insulin alone for treating pre-existing diabetes. Pioglitazone resulted in a lower HbA1c level (mean ± SD) (-1.21 ± 1.2 versus 0.39 ± 1%, P < 0.001) but had no effects on fasting blood glucose (6.58 ± 2.71 versus 7.28 ± 2.78 mmol/L, P = 0.14 ), and change in creatinine (3.54 ± 15.03 versus 10.61 ± 18.56 mmol/L, P = 0.53) and minimal adverse effects (no episodes of hypoglycaemia, three dropped out due to mild to moderate lower extremity oedema, cyclosporin levels were not affected).
Authors' conclusions: Evidence concerning the efficacy and safety of glucose-lowering agents for treating pre-existing and new-onset diabetes in kidney transplant recipients is limited. Existing studies examine more intensive versus less intensive insulin therapy, and the use of DPP4 inhibitors and pioglitazone. The safety and efficacy of more intensive compared to less intensive insulin therapy is very uncertain and the safety and efficacy of DPP4 inhibitors and pioglitazone is uncertain, due to data being limited and of poor quality. Additional RCTs are required to clarify the safety and efficacy of current glucose-lowering agents for kidney transplant recipients with diabetes.
Conflict of interest statement
Clement Lo: none known
Min Jun: none known
Sunil Badve: none known
Helen Pilmore: none known
Sarah White: none known
Carmel Hawley has received fees from Amgen, Shire, Roche, Abbott, Bayer, Fresenius, Baxter, Gambro, Janssen‐Cilag and Genzyme in relation to consultancy, speakers' fees, education, and grants for activities unrelated to this review
Alan Cass: Chronic kidney disease Advisory Board for Merck; receiving grant support from Baxter, Servier, Roche, and Gambro; and receiving lecture fees and travel reimbursements from Servier, Amgen, and Fresenius. The George Institute for Global Health conducted the ADVANCE Study, which was supported by a grant from Servier
Vlado Perkovic reports being a member of steering committees for and receiving honoria for scientific presentations from Boehringer Ingleheim, being a consultant and receiving honoraria for speaking at scientific symposia for Eli Lilly, receiving grants from and being a steering committee member and advisory board member for Janssen, being an advisory board member and receiving speaking honoraria from Astra Zeneca, receiving personal fees from Merck, and receiving speaking honoraria from Novo Nordisk.
Sophia Zoungas has previously received speaker honoraria from Servier, MSD, Sanofi Aventis, Eli Lilly, Astra Zeneca, BMS, Takeda and Janssen. She has previously served on external advisory boards for MSD, Novo Nordisk, Sanofi Aventis, Amgen, Novartis, Janssen, Astra Zeneca and Eli Lilly.
Figures
References
References to studies included in this review
-
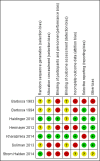
- Barbosa J, Connett J, Fryd D, Sutherland D, Rao V, Anderson R, et al. The Minnesota diabetes complications clinical trial. The first three years. Acta Diabetologica Latina 1983;20(2):165‐71. [MEDLINE: ] - PubMed
- Barbosa J, Johnson S. Severe hypoglycemia during maximized insulin treatment of diabetes in a randomized clinical trial. Diabetes Care 1983;6(1):62‐3. [MEDLINE: ] - PubMed
-
- Barbosa J, Steffes MW, Sutherland DE, Connett JE, Rao KV, Mauer SM. Effect of glycemic control on early diabetic renal lesions. A 5‐year randomized controlled clinical trial of insulin‐dependent diabetic kidney transplant recipients. JAMA 1994;272(8):600‐6. [MEDLINE: ] - PubMed
- Chang SS, Luiza M, Caramori A, Steffes MW, Barbosa J, Mauer M. Effect of cyclosporine (CSA) on early diabetic renal lesions in type 1 diabetic kidney transplant (TX) recipients [abstract]. Journal of the American Society of Nephrology 2000;11(Sept):113A. [CENTRAL: CN‐00550411]
-
- Haidinger M, Werzowa J, Hecking M, Antlanger M, Stemer G, Pleiner J, et al. Efficacy and safety of vildagliptin in new‐onset diabetes after kidney transplantation‐‐a randomized, double‐blind, placebo‐controlled trial. American Journal of Transplantation 2014;14(1):115‐23. [MEDLINE: ] - PubMed
- Haidinger M, Werzowa J, Hecking M, Pacini G, Antlanger M, Kovarik J. Efficacy and safety of vildagliptin in newly diagnosed diabetes after kidney transplantation: Results from the Vienna VINODAT trial [abstract]. Wiener Klinische Wochenschrift 2013;125(2 Suppl 1):S19‐20. [EMBASE: 71350674]
- Haidinger M, Werzowa J, Hecking M, Stemer G, Pleiner J, Kopecky C, et al. Efficacy and safety of vildagliptin in newly diagnosed diabetes after kidney transplantation [abstract no: O308]. Transplant International 2013;26(Suppl 2):175. [EMBASE: 71359610]
- Haidinger M, Werzowa J, Hecking M, Stemer G, Pleiner J, Kopecky C, et al. Vildagliptin in new‐onset diabetes after kidney transplantation is safe and efficient‐results from the Vienna VINODAT trial [abstract]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i77. [EMBASE: 71075201]
- Haidinger M, Werzowa J, Voigt HC, Pleiner J, Stemer G, Hecking M, et al. A randomized, placebo‐controlled, double‐blind, prospective trial to evaluate the effect of vildagliptin in new‐onset diabetes mellitus after kidney transplantation. Trials [Electronic Resource] 2010;11:91. [MEDLINE: ] - PMC - PubMed
-
- Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger L, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes. Journal of Clinical Endocrinology & Metabolism 2012;97(12):4399‐406. [MEDLINE: ] - PubMed
- Hermayer KL, Egidi MF, Finch NJ, Baliga P, Lin A, Kettinger LD, et al. A randomized controlled trial to evaluate the effect of glycemic control on renal transplantation outcomes [abstract no: 171‐OR]. Diabetes 2011;60(Suppl 1):A47. [EMBASE: 70627955] - PubMed
- Li P, Hunt KJ, Taber DJ, Carter RE, Kettinger L, Luttrell D, et al. Inflammatory biomarkers, glycemic variability, hypoglycemia, and renal transplant outcomes: results of a randomized controlled trial. Transplantation 2014;98(6):632‐9. [MEDLINE: ] - PubMed
-
- Kharazmkia A, Ahmadpoor P, Ziaie S, Salamzadeh J, Pour‐Reza‐Gholi F, Khoshdel A, et al. Effects of pioglitazone on blood glucose and inflammatory markers of diabetic kidney transplant patients: a randomized controlled trial. Iranian Journal of Kidney Diseases 2014;8(5):408‐16. [MEDLINE: ] - PubMed
References to studies excluded from this review
-
- Han SJ, Hur KY, Kim YS, Kang ES, Kim SI, Kim MS, et al. Effects of pioglitazone on subclinical atherosclerosis and insulin resistance in nondiabetic renal allograft recipients. Nephrology Dialysis Transplantation 2010;25(3):976‐84. [MEDLINE: ] - PubMed
-
- Hecking M, Haidinger M, Doller D, Werzowa J, Tura A, Zhang J, et al. Early basal insulin therapy decreases new‐onset diabetes after renal transplantation. Journal of the American Society of Nephrology 2012;23(4):739‐49. [MEDLINE: ] - PMC - PubMed
- Hecking M, Haidinger M, Doller D, Werzowa J, Tura A, Zhang J, et al. Early basal insulin therapy decreases new‐onset diabetes after renal transplantation by protecting endogenous insulin secretion [abstract no: MO‐008]. Transplant International 2011;24(Suppl 2):108. [EMBASE: 70527443]
- Hecking M, Werzowa J, Haidinger M, Do D, Pacini G, Saemann M. Early basal insulin therapy prevents new‐onset diabetes after transplantation by improving endogenous insulin secretion [abstract]. Diabetes 2011;60:A19‐20. [EMBASE: 70627857]
-
- Kim YS, Kim MS, Kim SI, Lim SK, Lee HY, Han DS, et al. Post‐transplantation diabetes is better controlled after conversion from prednisone to deflazacort: a prospective trial in renal transplants. Transplant International 1997;10(3):197‐201. [MEDLINE: ] - PubMed
-
- Kaisar M, Armstrong K, Prins J, Marwick T, Johnson D, Hawley C, et al. The impact of aggressive cardiovascular risk modification on carotid intima media thickness and brachial artery reactivity in renal transplant recipients [abstract no: 132]. Nephrology 2008;13(Suppl 3):A134. [CENTRAL: CN‐00766731]
- Kaisar MO, Armstrong K, Hawley C, Campbell S, Mudge D, Johnson DW, et al. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: baseline results from the LANDMARK 2 study. BMC Nephrology 2009;10:29. [MEDLINE: ] - PMC - PubMed
-
- Orazio LK, Isbel NM, Armstrong KA, Tarnarskyj J, Johnson DW, Hale RE, et al. Evaluation of dietetic advice for modification of cardiovascular disease risk factors in renal transplant recipients. Journal of Renal Nutrition 2011;21(6):462‐71. [MEDLINE: ] - PubMed
References to studies awaiting assessment
-
- Arashnia R, Roohi‐Gilani K, Karimi‐Sari H, Nikjoo N, Bahramifar A. Effect of pioglitazone therapy on high sensitive C‐reactive protein and lipid profile in diabetic patients with renal transplantation: a randomize clinical trial. Journal of Nephropathology 2015;4(2):48‐53. [MEDLINE: ] - PMC - PubMed
-
- Gross P. Influence of pioglitazone for renal transplant function in diabetics ‐ a double blind randomised placebo controlled cross over study. www.clinicaltrials.gov/ct2/show/NCT00507494 (accessed 19 October 2016).
Additional references
-
- American Diabetes Association. Standards of medical care in diabetes ‐ 2015. Diabetes Care 2015;38(Suppl 1):S1‐S94. - PubMed
-
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560‐72. [MEDLINE: ] - PubMed
-
- Bash LD, Selvin E, Steffes M, Coresh J, Astor BC. Poor glycemic control in diabetes and the risk of incident chronic kidney disease even in the absence of albuminuria and retinopathy: Atherosclerosis Risk in Communities (ARIC) Study. Archives of Internal Medicine 2008;168(22):2440‐7. [PUBMED: 19064828] - PMC - PubMed
-
- Burroughs TE, Swindle J, Takemoto S, Lentine KL, Machnicki G, Irish WD, et al. Diabetic complications associated with new‐onset diabetes mellitus in renal transplant recipients. Transplantation 2007;83(8):1027‐34. [PUBMED: 17452891] - PubMed
References to other published versions of this review
-
- Jun M, Lo C, Badve SV, Pilmore H, White SL, Hawley C, et al. Glucose lowering therapies for chronic kidney disease and kidney transplantation. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD009966] - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous