Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: Effect of a fourth dose of vaccine
- PMID: 28238631
- PMCID: PMC5665177
- DOI: 10.1016/j.vaccine.2017.02.021
Four-year persistence of type-specific immunity after quadrivalent human papillomavirus vaccination in HIV-infected children: Effect of a fourth dose of vaccine
Abstract
Objective: Although HIV-infected children are recommended to receive quadrivalent human papillomavirus vaccine (QHPV) there is limited information on their response to QHPV. This study in HIV-infected children, evaluated the magnitude and duration of immune responses to QHPV. This report describes type-specific serum antibody responses over a 4-to-5year period after either 3 or 4 doses of QHPV.
Design/methods: HIV-infected children, ages 7-to-11years, received 3 doses of QHPV (n=96) or placebo (n=30). At 72weeks QHPV recipients received a fourth dose (n=84), while placebo recipients began the 3-dose QHPV schedule (n=27). HPV serotype-specific antibody was determined, by competitive Luminex immunoassay (cLIA) and IgG Luminex immunoassay, at 2, 3.5, and 4-to-5years after the last dose of QHPV in each treatment arm.
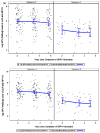
Results: At 4-to-5years after the last dose of QHPV, antibody titers were significantly higher in 4-dose than in 3-dose group. However, the proportion of vaccinees with a seroresponse in the cLIA assay was not different between the two groups (86-93% for HPV types 6, 11, and 16, and 64% for HPV type 18). These results were very similar to the seroresponse rate in these HIV-infected children at 1month after completing vaccination.
Conclusions: Children with well-controlled HIV infection who receive 3 doses of the QHPV vaccine maintain seropositivity and antibody levels that are generally similar to children of the same age who are not HIV-infected. Antibody titer correlated strongly with low log HIV RNA, low CD8%, and high CD4%. Additionally, a fourth dose of vaccine in HIV-infected children produces a marked rise in antibody characteristic of an anamnestic response and persistence of high antibody levels. Study identification: IMPAACT P1085 (V501-021). CLINICALTRIALS.GOV identifier: NCT01206556.
Keywords: Antibody response; Human papillomavirus vaccine; Pediatric HIV infection.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
M.J. Levin (chair) and A. Weinberg (immunologist) were members of the core protocol team supported by research grants from the sponsor and M.J. Levin is a consultant to the sponsor.
A Saah is an employee of the sponsor and may hold stock and/or stock options from the sponsor.
Figures

References
-
- Clifford G, Franceschi S, Diaz M, Munoz N, Villa LL. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24(Suppl 3):S26–34. - PubMed
-
- Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D. Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol. 2009;10(12):1152–9. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

