Tuned SMC Arms Drive Chromosomal Loading of Prokaryotic Condensin
- PMID: 28238653
- PMCID: PMC5344682
- DOI: 10.1016/j.molcel.2017.01.026
Tuned SMC Arms Drive Chromosomal Loading of Prokaryotic Condensin
Abstract
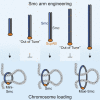
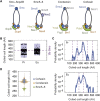
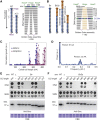
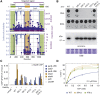
SMC proteins support vital cellular processes in all domains of life by organizing chromosomal DNA. They are composed of ATPase "head" and "hinge" dimerization domains and a connecting coiled-coil "arm." Binding to a kleisin subunit creates a closed tripartite ring, whose ∼47-nm-long SMC arms act as barrier for DNA entrapment. Here, we uncover another, more active function of the bacterial Smc arm. Using high-throughput genetic engineering, we resized the arm in the range of 6-60 nm and found that it was functional only in specific length regimes following a periodic pattern. Natural SMC sequences reflect these length constraints. Mutants with improper arm length or peptide insertions in the arm efficiently target chromosomal loading sites and hydrolyze ATP but fail to use ATP hydrolysis for relocation onto flanking DNA. We propose that SMC arms implement force transmission upon nucleotide hydrolysis to mediate DNA capture or loop extrusion.
Keywords: ParB; SMC; Smc/ScpAB; chromosome segregation; cohesin; coiled coil; condensin; heptad repeat; kleisin; periodicity.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Atkinson S.J., Stewart M. Molecular basis of myosin assembly: coiled-coil interactions and the role of charge periodicities. J. Cell Sci. Suppl. 1991;14:7–10. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

