Cullin E3 Ligase Activity Is Required for Myoblast Differentiation
- PMID: 28238764
- PMCID: PMC5395100
- DOI: 10.1016/j.jmb.2017.02.012
Cullin E3 Ligase Activity Is Required for Myoblast Differentiation
Abstract
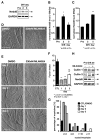
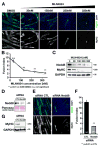
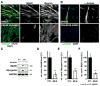
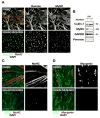
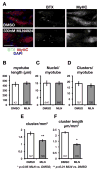
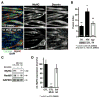
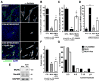
The role of cullin E3-ubiquitin ligases for muscle homeostasis is best known during muscle atrophy, as the cullin-1 substrate adaptor atrogin-1 is among the most well-characterized muscle atrogins. We investigated whether cullin activity was also crucial during terminal myoblast differentiation and aggregation of acetylcholine receptors for the establishment of neuromuscular junctions in vitro. The activity of cullin E3-ligases is modulated through post-translational modification with the small ubiquitin-like modifier nedd8. Using either the Nae1 inhibitor MLN4924 (Pevonedistat) or siRNA against nedd8 in early or late stages of differentiation on C2C12 myoblasts, and primary satellite cells from mouse and human, we show that cullin E3-ligase activity is necessary for each step of the muscle cell differentiation program in vitro. We further investigate known transcriptional repressors for terminal muscle differentiation, namely ZBTB38, Bhlhe41, and Id1. Due to their identified roles for terminal muscle differentiation, we hypothesize that the accumulation of these potential cullin E3-ligase substrates may be partially responsible for the observed phenotype. MLN4924 is currently undergoing clinical trials in cancer patients, and our experiments highlight concerns on the homeostasis and regenerative capacity of muscles in these patients who often experience cachexia.
Keywords: E3-ubiquitin ligase; MLN4924; cullin; muscle development.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Muscle-specific E3 ubiquitin ligases are involved in muscle atrophy of cancer cachexia: an in vitro and in vivo study.Oncol Rep. 2015 May;33(5):2261-8. doi: 10.3892/or.2015.3845. Epub 2015 Mar 9. Oncol Rep. 2015. PMID: 25760630
-
The E3 ubiquitin ligase TRIM32 regulates myoblast proliferation by controlling turnover of NDRG2.Hum Mol Genet. 2015 May 15;24(10):2873-83. doi: 10.1093/hmg/ddv049. Epub 2015 Feb 20. Hum Mol Genet. 2015. PMID: 25701873 Free PMC article.
-
Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells.Sci Rep. 2016 Apr 11;6:24218. doi: 10.1038/srep24218. Sci Rep. 2016. PMID: 27063292 Free PMC article.
-
Cullin 5-RING E3 ubiquitin ligases, new therapeutic targets?Biochimie. 2016 Mar;122:339-47. doi: 10.1016/j.biochi.2015.08.003. Epub 2015 Aug 4. Biochimie. 2016. PMID: 26253693 Review.
-
Ubiquitin ligases and medulloblastoma: genetic markers of the four consensus subgroups identified through transcriptome datasets.Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165839. doi: 10.1016/j.bbadis.2020.165839. Epub 2020 May 20. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32445667 Review.
Cited by
-
Neddylation Regulates Class IIa and III Histone Deacetylases to Mediate Myoblast Differentiation.Int J Mol Sci. 2021 Sep 1;22(17):9509. doi: 10.3390/ijms22179509. Int J Mol Sci. 2021. PMID: 34502418 Free PMC article.
-
Altered contractility, Ca2+ transients, and cell morphology seen in a patient-specific iPSC-CM model of Ebstein's anomaly with left ventricular noncompaction.Am J Physiol Heart Circ Physiol. 2023 Jul 1;325(1):H149-H162. doi: 10.1152/ajpheart.00658.2022. Epub 2023 May 19. Am J Physiol Heart Circ Physiol. 2023. PMID: 37204873 Free PMC article.
-
Cullin-3-RING ubiquitin ligase activity is required for striated muscle function in mice.J Biol Chem. 2018 Jun 8;293(23):8802-8811. doi: 10.1074/jbc.RA118.002104. Epub 2018 Apr 13. J Biol Chem. 2018. PMID: 29653945 Free PMC article.
-
6-Bromoindirubin-3'-oxime intercepts GSK3 signaling to promote and enhance skeletal muscle differentiation affecting miR-206 expression in mice.Sci Rep. 2019 Dec 2;9(1):18091. doi: 10.1038/s41598-019-54574-4. Sci Rep. 2019. PMID: 31792344 Free PMC article.
-
Defective Gating and Proteostasis of Human ClC-1 Chloride Channel: Molecular Pathophysiology of Myotonia Congenita.Front Neurol. 2020 Feb 11;11:76. doi: 10.3389/fneur.2020.00076. eCollection 2020. Front Neurol. 2020. PMID: 32117034 Free PMC article. Review.
References
-
- Blaauw B, Schiaffino S, Reggiani C. Mechanisms modulating skeletal muscle phenotype. Compr Physiol. 2013;3:1645–87. - PubMed
-
- Blondelle J, Lange S. Cardiac Cytoarchitecture: How to Maintain a Working Heart Waste—Disposal and Recycling in Cardiomyocytes. In: Ehler E, editor. Cardiac Cytoarchitecture. 1. Springer; 2015. pp. 245–309.
-
- Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

