Pyrrolysyl-tRNA synthetase, an aminoacyl-tRNA synthetase for genetic code expansion
- PMID: 28239189
- PMCID: PMC5321558
- DOI: 10.5562/cca2825
Pyrrolysyl-tRNA synthetase, an aminoacyl-tRNA synthetase for genetic code expansion
Abstract
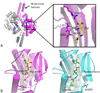
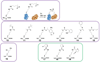
Genetic code expansion (GCE) has become a central topic of synthetic biology. GCE relies on engineered aminoacyl-tRNA synthetases (aaRSs) and a cognate tRNA species to allow codon reassignment by co-translational insertion of non-canonical amino acids (ncAAs) into proteins. Introduction of such amino acids increases the chemical diversity of recombinant proteins endowing them with novel properties. Such proteins serve in sophisticated biochemical and biophysical studies both in vitro and in vivo, they may become unique biomaterials or therapeutic agents, and they afford metabolic dependence of genetically modified organisms for biocontainment purposes. In the Methanosarcinaceae the incorporation of the 22nd genetically encoded amino acid, pyrrolysine (Pyl), is facilitated by pyrrolysyl-tRNA synthetase (PylRS) and the cognate UAG-recognizing tRNAPyl. This unique aaRS•tRNA pair functions as an orthogonal translation system (OTS) in most model organisms. The facile directed evolution of the large PylRS active site to accommodate many ncAAs, and the enzyme's anticodon-blind specific recognition of the cognate tRNAPyl make this system highly amenable for GCE purposes. The remarkable polyspecificity of PylRS has been exploited to incorporate >100 different ncAAs into proteins. Here we review the Pyl-OT system and selected GCE applications to examine the properties of an effective OTS.
Keywords: genetic code expansion; non-canonical amino acid; pyrrolysyl-tRNA synthetase; stop codon suppression; synthetic biology; tRNAPyl.
Figures
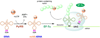
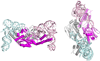

Similar articles
-
tRNAPyl: Structure, function, and applications.RNA Biol. 2018;15(4-5):441-452. doi: 10.1080/15476286.2017.1356561. Epub 2017 Sep 13. RNA Biol. 2018. PMID: 28837402 Free PMC article. Review.
-
Update of the Pyrrolysyl-tRNA Synthetase/tRNAPyl Pair and Derivatives for Genetic Code Expansion.J Bacteriol. 2023 Feb 22;205(2):e0038522. doi: 10.1128/jb.00385-22. Epub 2023 Jan 25. J Bacteriol. 2023. PMID: 36695595 Free PMC article. Review.
-
Pyrrolysyl-tRNA synthetase-tRNA(Pyl) structure reveals the molecular basis of orthogonality.Nature. 2009 Feb 26;457(7233):1163-7. doi: 10.1038/nature07611. Epub 2008 Dec 31. Nature. 2009. PMID: 19118381 Free PMC article.
-
Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool.Biochim Biophys Acta. 2014 Jun;1844(6):1059-70. doi: 10.1016/j.bbapap.2014.03.002. Epub 2014 Mar 12. Biochim Biophys Acta. 2014. PMID: 24631543 Free PMC article. Review.
-
Mutually orthogonal pyrrolysyl-tRNA synthetase/tRNA pairs.Nat Chem. 2018 Aug;10(8):831-837. doi: 10.1038/s41557-018-0052-5. Epub 2018 May 28. Nat Chem. 2018. PMID: 29807989 Free PMC article.
Cited by
-
Xenobiology for the Biocontainment of Synthetic Organisms: Opportunities and Challenges.Life (Basel). 2024 Aug 10;14(8):996. doi: 10.3390/life14080996. Life (Basel). 2024. PMID: 39202738 Free PMC article. Review.
-
Chemically Acylated tRNAs are Functional in Zebrafish Embryos.J Am Chem Soc. 2023 Feb 1;145(4):2414-2420. doi: 10.1021/jacs.2c11452. Epub 2023 Jan 20. J Am Chem Soc. 2023. PMID: 36669466 Free PMC article.
-
Expanding the Genetic Code of Xenopus laevis Embryos.ACS Chem Biol. 2024 Feb 16;19(2):516-525. doi: 10.1021/acschembio.3c00686. Epub 2024 Jan 26. ACS Chem Biol. 2024. PMID: 38277773 Free PMC article.
-
Crystal Structure of Pyrrolysyl-tRNA Synthetase from a Methanogenic Archaeon ISO4-G1 and Its Structure-Based Engineering for Highly-Productive Cell-Free Genetic Code Expansion with Non-Canonical Amino Acids.Int J Mol Sci. 2023 Mar 26;24(7):6256. doi: 10.3390/ijms24076256. Int J Mol Sci. 2023. PMID: 37047230 Free PMC article.
-
tRNAPyl: Structure, function, and applications.RNA Biol. 2018;15(4-5):441-452. doi: 10.1080/15476286.2017.1356561. Epub 2017 Sep 13. RNA Biol. 2018. PMID: 28837402 Free PMC article. Review.
References
-
- Ambrogelly A, Palioura S, Söll D. Nat. Chem. Biol. 2007;3:29–35. - PubMed
-
- Park HS, Hohn MJ, Umehara T, Guo LT, Osborne EM, Benner J, Noren CJ, Rinehart J, Söll D. Science. 2011;333:1151–1154. - PMC - PubMed
- Rogerson DT, Sachdeva A, Wang K, Haq T, Kazlauskaite A, Hancock SM, Huguenin-Dezot N, Muqit MM, Fry AM, Bayliss R, Chin JW. Nat. Chem. Biol. 2015;11:496–503. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
