The Rise of Radicals in Bioinorganic Chemistry
- PMID: 28239191
- PMCID: PMC5321091
- DOI: 10.1002/ijch.201600069
The Rise of Radicals in Bioinorganic Chemistry
Abstract
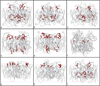
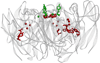
Prior to 1950, the consensus was that biological transformations occurred in two-electron steps, thereby avoiding the generation of free radicals. Dramatic advances in spectroscopy, biochemistry, and molecular biology have led to the realization that protein-based radicals participate in a vast array of vital biological mechanisms. Redox processes involving high-potential intermediates formed in reactions with O2 are particularly susceptible to radical formation. Clusters of tyrosine (Tyr) and tryptophan (Trp) residues have been found in many O2-reactive enzymes, raising the possibility that they play an antioxidant protective role. In blue copper proteins with plastocyanin-like domains, Tyr/Trp clusters are uncommon in the low-potential single-domain electron-transfer proteins and in the two-domain copper nitrite reductases. The two-domain muticopper oxidases, however, exhibit clusters of Tyr and Trp residues near the trinuclear copper active site where O2 is reduced. These clusters may play a protective role to ensure that reactive oxygen species are not liberated during O2 reduction.
Keywords: Electron transfer; radicals; tryptophan; tyrosine.
Figures


References
-
- Bertini I, Gray HB, Stiefel EI, Valentine JS. Biological Inorganic Chemistry - Structure and Reactivity. University Science Books, Sausalito: California; 2007.
-
- Michaelis L. In: Currents in Biochemical Research. Green DE, editor. New York: Interscience Publishers, Inc.; 1946. pp. 207–227.
-
- Commoner B, Townsend J, Pake GE. Nature. 1954;174:689–691. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
