Poly(hydroxy alkanoate)s in Medical Applications
- PMID: 28239227
- PMCID: PMC5321601
- DOI: 10.15255/CABEQ.2014.2261
Poly(hydroxy alkanoate)s in Medical Applications
Abstract
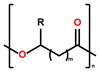
This review summarizes the state-of-the-art knowledge of the usage of poly(hydroxy alkanoate)s in medical and sanitary applications. Depending on the monomers incorporated into the polymers and copolymers, this class of polymers exhibits a broad range of (thermo-)plastic properties, enabling their processing by, e.g., solution casting or melt extrusion. In this review, strategies for the polymer analogous modification of these materials and their surfaces are highlighted and correlated with the potential applications of the corresponding materials and blends. While the commercial availability of purified PHAs is addressed in brief, special focus is put on the (bio-)degradability of these polymers and ways to influence the degradation mechanism and/or the duration of degradation.
Keywords: bio-degradation; medical application; poly(hydroxy alkanoate); polymer analogous modification; polymer processing.
Figures





Similar articles
-
Morphologies, Compatibilization and Properties of Immiscible PLA-Based Blends with Engineering Polymers: An Overview of Recent Works.Polymers (Basel). 2024 Jun 23;16(13):1776. doi: 10.3390/polym16131776. Polymers (Basel). 2024. PMID: 39000632 Free PMC article. Review.
-
Bio-Based and Biodegradable Polymeric Materials for a Circular Economy.Polymers (Basel). 2024 Oct 28;16(21):3015. doi: 10.3390/polym16213015. Polymers (Basel). 2024. PMID: 39518225 Free PMC article. Review.
-
Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications.ACS Biomater Sci Eng. 2021 Dec 13;7(12):5339-5362. doi: 10.1021/acsbiomaterials.1c00757. Epub 2021 Oct 14. ACS Biomater Sci Eng. 2021. PMID: 34649426 Free PMC article. Review.
-
Processing of (Co)Poly(2-oxazoline)s by Electrospinning and Extrusion from Melt and the Postprocessing Properties of the (Co)Polymers.Polymers (Basel). 2020 Feb 2;12(2):295. doi: 10.3390/polym12020295. Polymers (Basel). 2020. PMID: 32024273 Free PMC article.
-
Alginate-Based Bio-Composites and Their Potential Applications.J Funct Biomater. 2022 Aug 10;13(3):117. doi: 10.3390/jfb13030117. J Funct Biomater. 2022. PMID: 35997455 Free PMC article. Review.
Cited by
-
Bioinformatics Analysis of Metabolism Pathways of Archaeal Energy Reserves.Sci Rep. 2019 Jan 31;9(1):1034. doi: 10.1038/s41598-018-37768-0. Sci Rep. 2019. PMID: 30705313 Free PMC article.
-
Esterase-Cleavable 2D Assemblies of Magnetic Iron Oxide Nanocubes: Exploiting Enzymatic Polymer Disassembling To Improve Magnetic Hyperthermia Heat Losses.Chem Mater. 2019 Aug 13;31(15):5450-5463. doi: 10.1021/acs.chemmater.9b00728. Epub 2019 Jun 26. Chem Mater. 2019. PMID: 31631940 Free PMC article.
-
Sustainability Evaluation of Polyhydroxyalkanoate Production from Slaughterhouse Residues Utilising Emergy Accounting.Polymers (Basel). 2021 Dec 29;14(1):118. doi: 10.3390/polym14010118. Polymers (Basel). 2021. PMID: 35012140 Free PMC article.
-
Bio-Based Electrospun Fibers for Wound Healing.J Funct Biomater. 2020 Sep 22;11(3):67. doi: 10.3390/jfb11030067. J Funct Biomater. 2020. PMID: 32971968 Free PMC article. Review.
-
Resorbable implants in pediatric fracture treatment.Innov Surg Sci. 2018 May 29;3(2):119-125. doi: 10.1515/iss-2018-0006. eCollection 2018 Jun. Innov Surg Sci. 2018. PMID: 31579775 Free PMC article. Review.
References
-
- Lemoigne M. Produits de dehydration et de polymerisation de l’acide oxobutyrique. Bull Soc Chim Biol. 1926;8:770.
Grants and funding
LinkOut - more resources
Full Text Sources
