MicroPET Outperforms Beta-Microprobes in Determining Neuroreceptor Availability under Pharmacological Restriction for Cold Mass Occupancy
- PMID: 28239334
- PMCID: PMC5301012
- DOI: 10.3389/fnins.2017.00047
MicroPET Outperforms Beta-Microprobes in Determining Neuroreceptor Availability under Pharmacological Restriction for Cold Mass Occupancy
Abstract
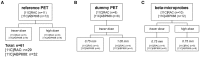
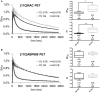
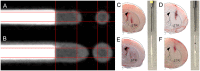
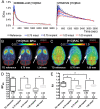
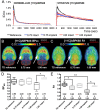
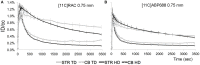
Both non-invasive micro-positron emission tomography (μPET) and in situ beta-microprobes have the ability to determine radiotracer kinetics and neuroreceptor availability in vivo. Beta-microprobes were proposed as a cost-effective alternative to μPET, but literature revealed conflicting results most likely due to methodological differences and inflicted tissue damage. The current study has three main objectives: (i) evaluate the theoretical advantages of beta-microprobes; (ii) perform μPET imaging to assess the impact of (beta-micro)probe implantation on relative tracer delivery (R1) and receptor occupancy (non-displaceable binding potential, BPND) in the rat brain; and (iii) investigate whether beta-microprobe recordings produce robust results when a pharmacological restriction for cold mass dose (tracer dose condition) is imposed. We performed acquisitions (n = 61) in naive animals, dummy probe implanted animals (outer diameter: 0.75 and 1.00 mm) and beta-microprobe implanted animals (outer diameter: 0.75 mm) using two different radiotracers with high affinity for the striatum: [11C]raclopride (n = 29) and [11C]ABP688 (n = 32). In addition, acquisitions were completed with or without an imposed restriction for cold mass occupancy. We estimated BPND and R1 values using the simplified reference tissue method (SRTM). [11C]raclopride dummy μPET BPND (0.75 mm: -13.01 ± 0.94%; 1.00 mm: -13.89 ± 1.20%) and R1 values (0.75 mm: -29.67 ± 4.94%; 1.00 mm: -39.07 ± 3.17%) significantly decreased at the implant side vs. the contralateral intact side. A similar comparison for [11C]ABP688 dummy μPET, demonstrated significantly (p < 0.05) decreased BPND (-19.09 ± 2.45%) and R1 values (-38.12 ± 6.58%) in the striatum with a 1.00 mm implant, but not with a 0.75 mm implant. Particularly in tracer dose conditions, despite lower impact of partial volume effects, beta-microprobes proved unfit to produce representative results due to tissue destruction associated with probe insertion. We advise to use tracer dose μPET to obtain accurate results concerning receptor availability and tracer delivery, keeping in mind associated partial volume effects for which it is possible to correct.
Keywords: [11C]ABP688; [11C]raclopride; beta-microprobe; rat brain; μPET.
Figures
References
-
- Alexoff D. L., Vaska P., Marsteller D., Gerasimov T., Li J., Logan J., et al. (2003). Reproducibility of 11C-raclopride binding in the rat brain measured with the microPET R4: effects of scatter correction and tracer specific activity. J. Nucl. Med. 44, 815–822. - PubMed
-
- Ametamey S. M., Treyer V., Streffer J., Wyss M. T., Schmidt M., Blagoev M., et al. (2007). Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J. Nucl. Med. 48, 247–252. - PubMed
-
- Balasse L., Märk J., Pain F., Genoux A., Fieux S., Lefebvre F., et al. (2014). PIXSIC: a wireless intracerebral radiosensitive probe in freely moving rats. Mol. Imaging 14, 484–489. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

