Informative Cues Facilitate Saccadic Localization in Blindsight Monkeys
- PMID: 28239342
- PMCID: PMC5300996
- DOI: 10.3389/fnsys.2017.00005
Informative Cues Facilitate Saccadic Localization in Blindsight Monkeys
Abstract
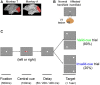
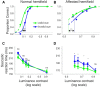
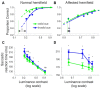
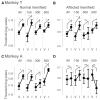
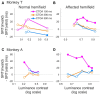
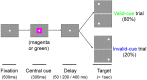
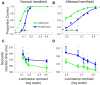
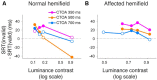
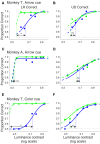
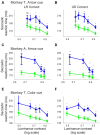
Patients with damage to the primary visual cortex (V1) demonstrate residual visual performance during laboratory tasks despite denying having a conscious percept. The mechanisms behind such performance, often called blindsight, are not fully understood, but the use of surgically-induced unilateral V1 lesions in macaque monkeys provides a useful animal model for exploring such mechanisms. For example, V1-lesioned monkeys localize stimuli in a forced-choice condition while at the same time failing to report awareness of identical stimuli in a yes-no detection condition, similar to human patients. Moreover, residual cognitive processes, including saliency-guided eye movements, bottom-up attention with peripheral non-informative cues, and spatial short-term memory, have all been demonstrated in these animals. Here we examined whether post-lesion residual visuomotor processing can be modulated by top-down task knowledge. We tested two V1-lesioned monkeys with a visually guided saccade task in which we provided an informative foveal pre-cue about upcoming target location. Our monkeys fixated while we presented a leftward or rightward arrow (serving as a pre-cue) superimposed on the fixation point (FP). After various cue-target onset asynchronies (CTOAs), a saccadic target (of variable contrast across trials) was presented either in the affected (contra-lesional) or seeing (ipsi-lesional) hemifield. Critically, target location was in the same hemifield that the arrow pre-cue pointed towards in 80% of the trials (valid-cue trials), making the cue highly useful for task performance. In both monkeys, correct saccade reaction times were shorter during valid than invalid trials. Moreover, in one monkey, the ratio of correct saccades towards the affected hemifield was higher during valid than invalid trials. We replicated both reaction time and correct ratio effects in the same monkey using a symbolic color cue. These results suggest that V1-lesion monkeys can use informative cues to localize stimuli in the contra-lesional hemifield, consistent with reports of a human blindsight subject being able to direct attention in cueing paradigms. Because the superior colliculus (SC) may contribute to residual visual capabilities after V1 lesions, and because this structure is important for controlling attentional resources, we hypothesize that our results reflect, among others, SC involvement in integrating top-down task knowledge for guiding orienting behavior.
Keywords: Posner cueing; blindsight; covert visual attention; eye movements; macaque monkeys.
Figures

References
-
- Aizawa H., Wurtz R. H. (1998). Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. J. Neurophysiol. 79, 2082–2096. - PubMed
-
- Bowman E. M., Brown V. J., Kertzman C., Schwarz U., Robinson D. L. (1993). Covert orienting of attention in macaques. I. Effects of behavioral context. J. Neurophysiol. 70, 431–443. - PubMed
-
- Campion J., Latto R., Smith Y. M. (1983). Is blindsight an effect of scattered light, spared cortex and near-threshold vision? Behav. Brain Sci. 6, 423–448. 10.1017/s0140525x00016861 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

