Hydrogen Peroxide Pretreatment Mitigates Cadmium-Induced Oxidative Stress in Brassica napus L.: An Intrinsic Study on Antioxidant Defense and Glyoxalase Systems
- PMID: 28239385
- PMCID: PMC5300965
- DOI: 10.3389/fpls.2017.00115
Hydrogen Peroxide Pretreatment Mitigates Cadmium-Induced Oxidative Stress in Brassica napus L.: An Intrinsic Study on Antioxidant Defense and Glyoxalase Systems
Abstract
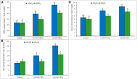
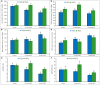
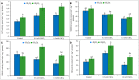
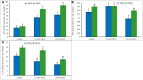
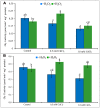
Cadmium (Cd) is considered as one of the most toxic metals for plant growth and development. In the present study, we investigated the role of externally applied hydrogen peroxide (H2O2) in regulating the antioxidant defense and glyoxalase systems in conferring Cd-induced oxidative stress tolerance in rapeseed (Brassica napus L.). Seedlings were pretreated with 50 μM H2O2 for 24 h. These pretreated seedlings as well as non-pretreated seedlings were grown for another 48 h at two concentrations of CdCl2 (0.5 and 1.0 mM). Both the levels of Cd increased MDA and H2O2 levels and lipoxygenase activity while ascorbate (AsA) declined significantly. However, reduced glutathione (GSH) content showed an increase at 0.5 mM CdCl2, but glutathione disulfide (GSSG) increased at any level of Cd with a decrease in GSH/GSSG ratio. The activities of ascorbate peroxidase (APX) and glutathione S-transferase (GST) upregulated due to Cd treatment in dose-dependent manners, while glutathione reductase (GR) and glutathione peroxidase (GPX) increased only at 0.5 mM CdCl2 and decreased at higher dose. The activity of monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), catalase (CAT), glyoxalase I (Gly I), and glyoxalase II (Gly II) decreased under Cd stress. On the other hand, H2O2 pretreated seedlings, when exposed to Cd, AsA and GSH contents and GSH/GSSG ratio increased noticeably. H2O2 pretreatment increased the activities of APX, MDHAR, DHAR, GR, GST, GPX, and CAT of Cd affected seedlings. Thus enhancement of both the non-enzymatic and enzymatic antioxidants helped to decrease the oxidative damage as indicated by decreased levels of H2O2 and MDA. The seedlings which were pretreated with H2O2 also showed enhanced glyoxalase system. The activities of Gly I, and Gly II and the content of GSH increased significantly due to H2O2 pretreatment in Cd affected seedlings, compared to the Cd-stressed plants without H2O2 pretreatment which were vital for methylglyoxal detoxification. So, the major roles of H2O2 were improvement of antioxidant defense system and glyoxalase system which protected plants from the damage effects of ROS and MG. The mechanism of H2O2 to induce antioxidant defense and glyoxalase system and improving physiology under stress condition is not known clearly which should be elucidated. The signaling roles of H2O2 and its interaction with other signaling molecules, phytohormones or other biomolecules and their roles in stress protection should be explored.
Keywords: abiotic stress; antioxidant defense; cross tolerance; metal toxicity; methylglyoxal; oxidative stress; signaling molecule.
Figures
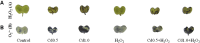
References
-
- Addinsoft (2016). XLSTAT V. 2016.04.32525: Data Analysis and Statistics Software for Microsoft Excel. Paris: Addinsoft.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

