Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions
- PMID: 28239429
- PMCID: PMC5299475
- DOI: 10.1002/cctc.201600996
Future Challenges in Heterogeneous Catalysis: Understanding Catalysts under Dynamic Reaction Conditions
Abstract
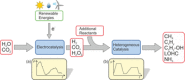
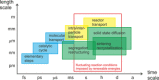
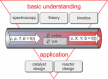
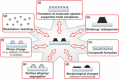
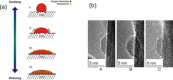
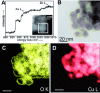
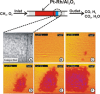
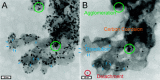
In the future, (electro-)chemical catalysts will have to be more tolerant towards a varying supply of energy and raw materials. This is mainly due to the fluctuating nature of renewable energies. For example, power-to-chemical processes require a shift from steady-state operation towards operation under dynamic reaction conditions. This brings along a number of demands for the design of both catalysts and reactors, because it is well-known that the structure of catalysts is very dynamic. However, in-depth studies of catalysts and catalytic reactors under such transient conditions have only started recently. This requires studies and advances in the fields of 1) operando spectroscopy including time-resolved methods, 2) theory with predictive quality, 3) kinetic modelling, 4) design of catalysts by appropriate preparation concepts, and 5) novel/modular reactor designs. An intensive exchange between these scientific disciplines will enable a substantial gain of fundamental knowledge which is urgently required. This concept article highlights recent developments, challenges, and future directions for understanding catalysts under dynamic reaction conditions.
Keywords: electrocatalysis; energy storage; heterogeneous catalysis; molecular modelling; operando spectroscopy.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
