Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy
- PMID: 28239462
- PMCID: PMC5319013
- DOI: 10.1186/s40425-017-0210-0
Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy
Abstract
Background: Immune checkpoint inhibitors, including antibodies against programmed death 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), are being used with increasing frequency for the treatment of cancer. Immune-related adverse events (irAEs) including colitis, dermatitis, and pneumonitis are well described, but less frequent events are now emerging with larger numbers of patients treated. Herein we describe the incidence and spectrum of thrombocytopenia following immune checkpoint inhibitor therapy and two severe cases of idiopathic thrombocytopenic purpura (ITP).
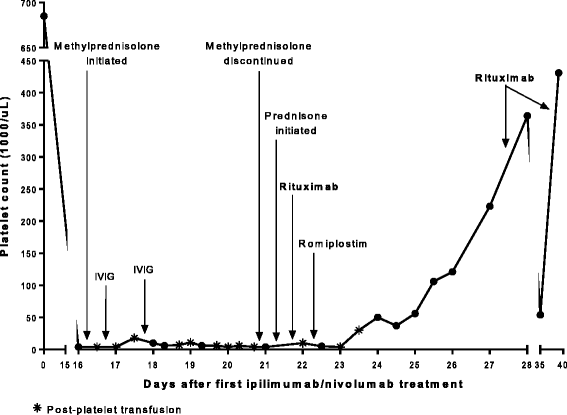
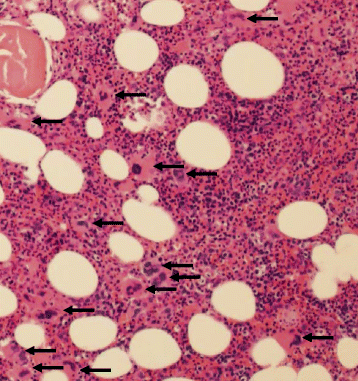
Case presentations: A 47-year-old female with recurrent BRAF mutant positive melanoma received combination anti-PD-1 and anti-CTLA-4. Two weeks later, she presented with mucosal bleeding, petechiae, and thrombocytopenia and was treated with standard therapy for ITP with steroids and intravenous immunoglobulin (IVIG). Her diagnosis was confirmed with bone marrow biopsy, and given the lack of treatment response, she was treated with rituximab. She began to have recovery and stabilization of her platelet count that ultimately allowed her to be retreated with PD-1 inhibition with no further thrombocytopenia. A second patient, a 45-year-old female with a BRAF wild-type melanoma, received anti-PD-1 monotherapy and became thrombocytopenic 43 days later. Three weeks of steroid treatment improved her platelet count, but thrombocytopenia recurred and required additional steroids. She later received anti-CTLA-4 monotherapy and developed severe ITP with intracranial hemorrhage. Her ITP resolved after treatment of prednisone, IVIG, and rituximab and discontinuation of checkpoint inhibition. In a retrospective chart review of 2360 patients with melanoma treated with checkpoint inhibitor therapy, <1% experienced thrombocytopenia following immune checkpoint inhibition, and of these, most had spontaneous resolution and did not require treatment.
Conclusions: Thrombocytopenia, especially ITP, induced by immune checkpoint inhibitors appears to be an uncommon irAE that is manageable with observation in mild cases and/or standard ITP treatment algorithms. In our series, the majority of patients had mild thrombocytopenia that resolved spontaneously or responded to standard corticosteroid regimens. However, in two severe cases, IVIG and rituximab, in addition to steroids, were required. Checkpoint inhibition was resumed successfully in the first patient but rechallenge was not tolerated by the second patient.
Keywords: CTLA-4; Checkpoint inhibitor; Immune thrombocytopenic purpura; Melanoma; PD-1; Thrombocytopenia.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
