Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody
- PMID: 28239472
- PMCID: PMC5320726
- DOI: 10.1186/s40425-017-0220-y
Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody
Abstract
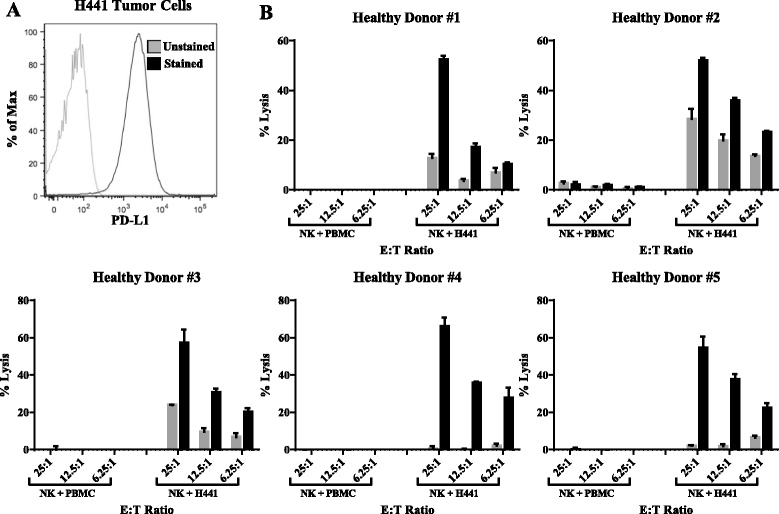
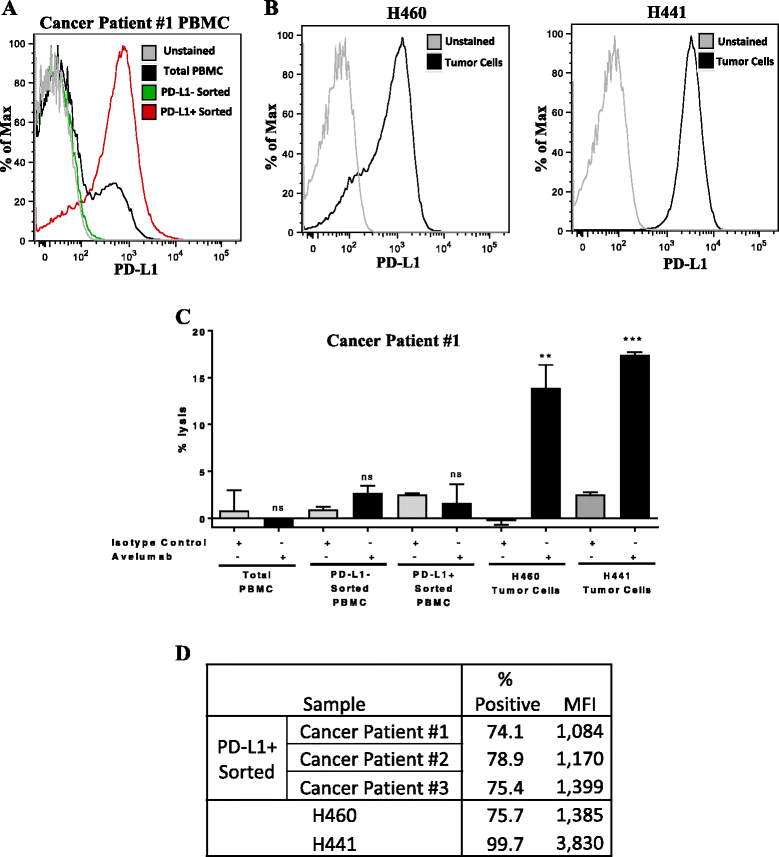
Background: Multiple anti-PD-L1/PD-1 checkpoint monoclonal antibodies (MAb) have shown clear evidence of clinical benefit. All except one have been designed or engineered to omit the possibility to mediate antibody-dependent cell-mediated cytotoxicity (ADCC) as a second potential mode of anti-tumor activity; the reason for this is the concern of lysis of PD-L1 positive immune cells. Avelumab is a fully human IgG1 MAb which has been shown in prior in vitro studies to mediate ADCC versus a range of human tumor cells, and clinical studies have demonstrated anti-tumor activity versus a range of human cancers. This study was designed to investigate the effect on immune cell subsets in the peripheral blood of cancer patients prior to and following multiple administrations of avelumab.
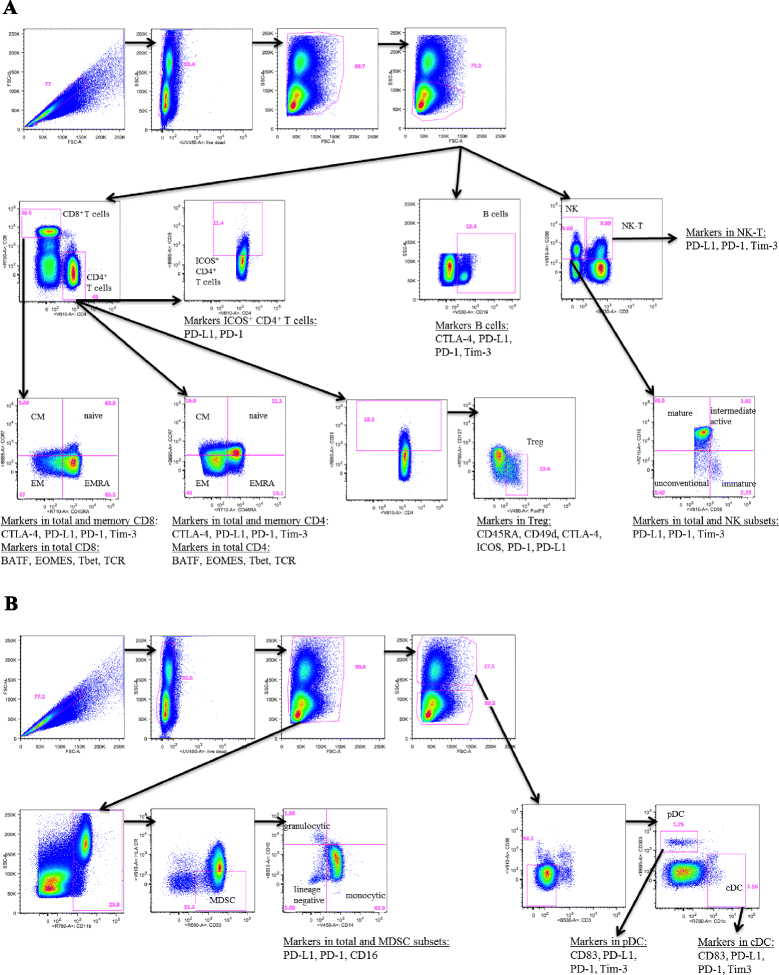
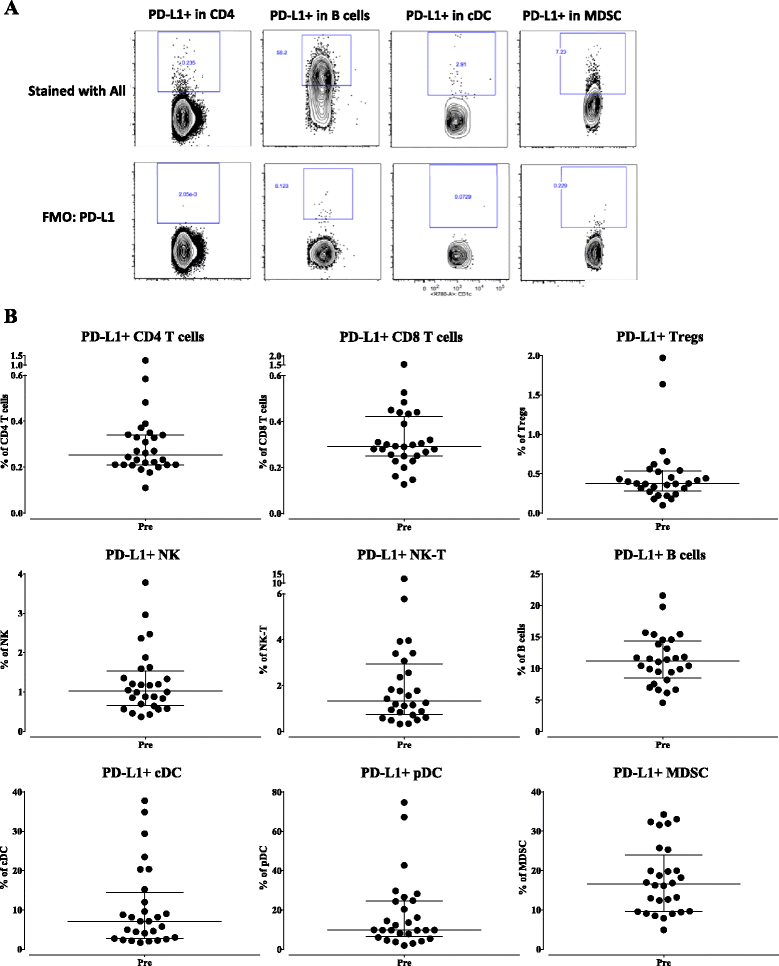
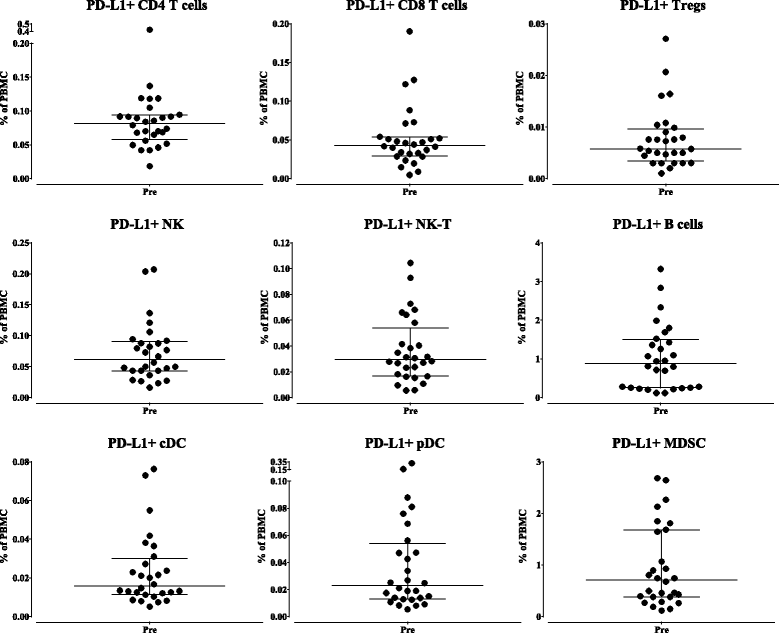
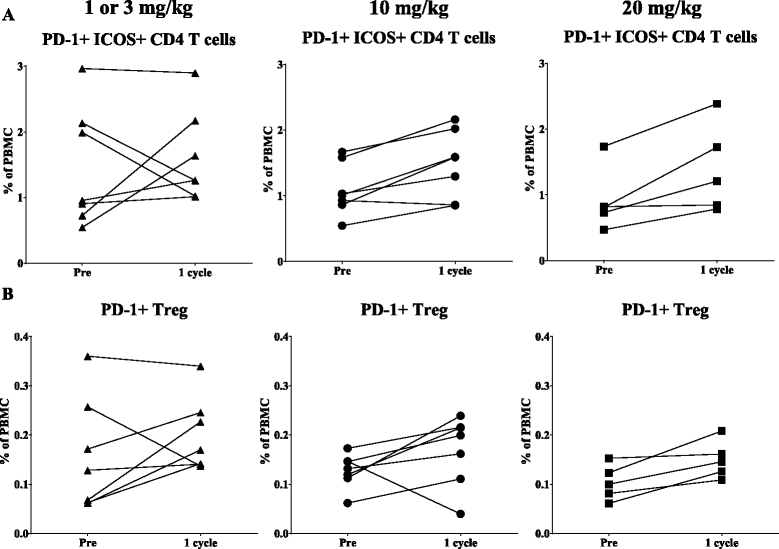
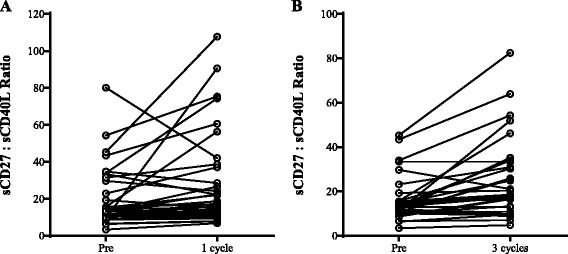
Methods: One hundred twenty-three distinct immune cell subsets in the peripheral blood of cancer patients (n = 28) in a phase I trial were analyzed by flow cytometry prior to and following one, three, and nine cycles of avelumab. Changes in soluble (s) CD27 and sCD40L in plasma were also evaluated. In vitro studies were also performed to determine if avelumab would mediate ADCC of PBMC.
Results: No statistically significant changes in any of the 123 immune cell subsets analyzed were observed at any dose level, or number of doses, of avelumab. Increases in the ratio of sCD27:sCD40L were observed, suggesting potential immune activation. Controlled in vitro studies also showed lysis of tumor cells by avelumab versus no lysis of PBMC from five donors.
Conclusions: These studies demonstrate the lack of any significant effect on multiple immune cell subsets, even those expressing PD-L1, following multiple cycles of avelumab. These results complement prior studies showing anti-tumor effects of avelumab and comparable levels of adverse events with avelumab versus other anti-PD-1/PD-L1 MAbs. These studies provide the rationale to further exploit the potential ADCC mechanism of action of avelumab as well as other human IgG1 checkpoint inhibitors.
Trial registration: ClinicalTrials.gov identifier: NCT01772004 (first received: 1/14/13; start date: January 2013) and NCT00001846 (first received date: 11/3/99; start date: August 1999).
Keywords: ADCC; Anti-PD-L1; Antibody-dependent cell-mediated cytotoxicity; Avelumab; Checkpoint inhibitor; Immune subsets; Immunotherapy; Peripheral immunome.
Figures
References
-
- Khanna S, et al. Malignant mesothelioma effusions are infiltrated by CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells within these effusions are susceptible to ADCC by the anti-PD-L1 antibody avelumab. J Thorac Oncol. 2016;11:1993–200. doi: 10.1016/j.jtho.2016.07.033. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
